In a clinical trial landscape where global, multi-center studies have become increasingly common, trial monitoring has become more complex. Regulations require that sponsors of medical device studies perform site monitoring to ensure that investigators are compliant with federal regulations, sponsor agreements, the investigational plan and the requirements set forth in the Investigational Review Board (IRB) approval of the study. In addition, sponsors are responsible for selecting qualified monitors to oversee the safety of trial participants.
Sponsors may appoint other individuals and groups, such as a Data Monitoring Committee (DMC) or Clinical Endpoint Committee (CEC), to ensure compliance and proper clinical trial monitoring. But what is the difference between a DMC and a CEC, and how do sponsors know what type of study oversight is needed?
Data Monitoring Committees
The FDA has issued guidance for clinical trial sponsors on the establishment and operation of clinical trial Data Monitoring Committees, also known as Data and Safety Monitoring Boards (DSMBs), Data and Safety Monitoring Committees (DSMCs), or Independent Data Monitoring Committee (IDMCs).[1] The guidance is intended to assist sponsors in determining when a DMC may be useful for study monitoring and how such committees should operate.
The FDA recommends that sponsors consider using a DMC when:1
- The study endpoint is such that a highly favorable or unfavorable result, including a finding of futility, at an interim analysis might ethically require early termination of the study
- There are a priori reasons for a safety concern, e.g., a particularly invasive procedure
- There is prior information suggesting the possibility of serious toxicity
- The study is being performed in a potentially fragile or vulnerable population
- The study is being performed in a population at an elevated risk of death or other serious outcomes
- The study is large, or long duration, and multi-center
A DMC is a group of individuals with relevant expertise that:
- Reviews accumulating data from an ongoing clinical trial on a regular basis
- Advises the sponsor on the continuing safety of clinical trial participants
- Provides recommendations to the sponsor on the ongoing validity and scientific merit of the study
Clinical Endpoint Committees
A Clinical Endpoint Committee (CEC) — also known as an adjudication committee or Endpoint Adjudication Committee (EAE) — is another type of oversight group that a sponsor may delegate to share the responsibilities of clinical monitoring. CECs are used for centralized adjudication of endpoints to help standardize outcomes and optimize data quality. These types of committees are most valuable when:
- Endpoints are subject to interpretation, e.g., clinical events
- Endpoints require the application of a complex definition
- The study cannot be blinded
- The study is expected to have a high enrollment or long duration
- Global or cultural differences are expected across study sites
- Endpoints of interest fall outside the therapeutic expertise of the investigator
- Data are needed to support DMC functions or adaptive study designs
CECs are typically comprised of an independent group of clinical and/or diagnostics experts who are responsible for:
- Centrally reviewing and classifying suspected safety and/or efficacy endpoints in a blinded, unbiased, confidential, and consensus-based manner
- Determining whether these endpoints meet protocol definitions
- Providing standardized endpoint outcomes for statistical analysis
- Classifying events as related to a study device and/or procedure (in medical device studies)
According to FDA Guidance, CECs should be blinded to treatment whenever possible, even if the trial in question is not blinded.1 In addition, the adjudication process should be designed to both preserve the independence of the CEC and prevent any undue bias that could impact its decision-making.[2] Of note, CECs do not perform interim analyses or make recommendations to the sponsor.
Comparing DMCs and CECs
Both DMCs and CECs are comprised of independent physicians who review study data. Both types of committees require charters that define their review process and responsibilities, as well as no conflict of interest declarations. However, the committees differ in their objectives and their structures:
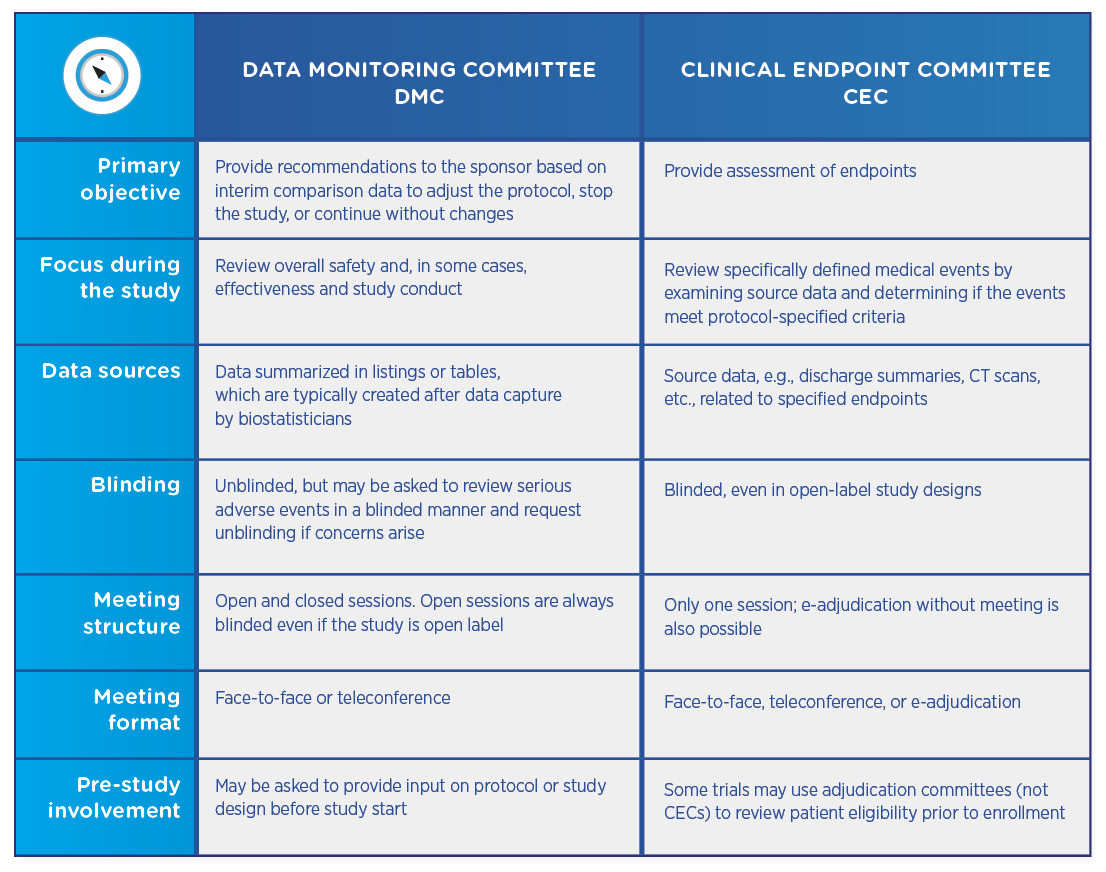
DMCs and CECs are not mutually exclusive, and a trial may utilize either committee or both. If both committees are involved, the CEC usually precedes the DMC, which then reviews already-adjudicated data to ensure that it is as accurate and unbiased as possible.
The decision to utilize a DMC or CEC should be made early in the clinical trial planning process, as the data exchange, coordination, and communication involved in operating and managing such committees adds complexity. Working with a CRO partner who understands when and how to leverage these oversight groups can help sponsors manage and operate these committees in an efficient manner that is customized to the nuances of their clinical trials.
[1] U.S. Food and Drug Administration. Guidance for Clinical Trial Sponsors: Establishment and Operation of Clinical Trial Data Monitoring Committees, March 2006. Available at https://www.fda.gov/downloads/RegulatoryInformation/Guidances/ucm127073.pdf.
[2] Tyner CA, et al. Establishment and Operation of Clinical Endpoint Committees: Best Practice for Implementation Across the Biopharmaceutical Industry, White Paper.
