As medical device regulations evolve and global, multi-center studies become increasingly common, a growing number of sponsors are turning to independent committees such as Data Monitoring Committees (DMCs) or Clinical Endpoint Committees (CECs) for assistance with clinical trial oversight. However, given that neither the U.S. Food and Drug Administration (FDA) nor the European Medicines Agency (EMA) typically require the use of these committees, sponsors may have questions about when and how these committees should be used.
Livia Hlavackova, MD, Ph.D., Senior Medical Director at Premier Research, was recently invited to discuss this topic at the 6th Annual Outsourcing in Clinical Trials Medical Devices Europe Conference 2019 held in Munich, Germany. In her presentation, “Charting the Course: Choosing the Right Committee for Your Medical Device Trial,” she explored the key responsibilities of — and distinctions between — DMCs and CECs and provided insight on how sponsors can select the most appropriate type of oversight for a particular study.
Here are three key takeaways from that presentation:
- Begin by understanding the objective and structure of each type of committee
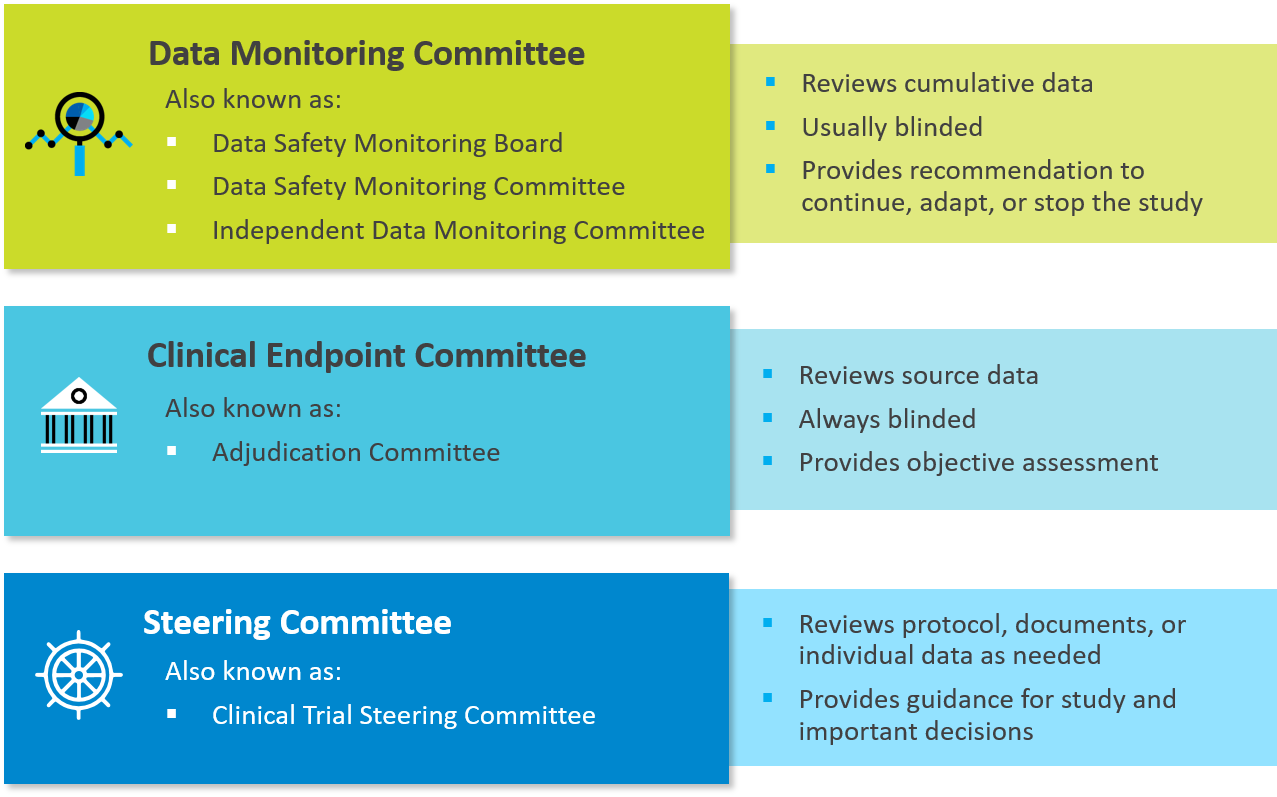
- Study-specific factors may influence committee selection
To be effective, independent committees should be customized to the nuances of a particular medical device trial.
A DMC may be appropriate if:
- the result of a study endpoint at an interim analysis might require early termination of the study
- there are known risks or serious toxicities associated with the treatment
- the study population is potentially fragile or vulnerable
- the study is large, of long duration, or involves multiple sites
A CEC may be suitable if:
- endpoints are subject to interpretation or require the application of a complex definition
- the study cannot be blinded
- the study is large, of long duration, or subject to potential global or cultural differences
- data are needed to support DMC functions or adaptive study designs
- Set the committee up for success
The decision to utilize an independent committee should be made as early as possible in the clinical trial planning. The data exchange, coordination, and communication involved in setting up and managing these committees adds a layer of complexity to the study, which may require additional time and resources. The figure below outlines the process involved in operating a DMC or CEC:
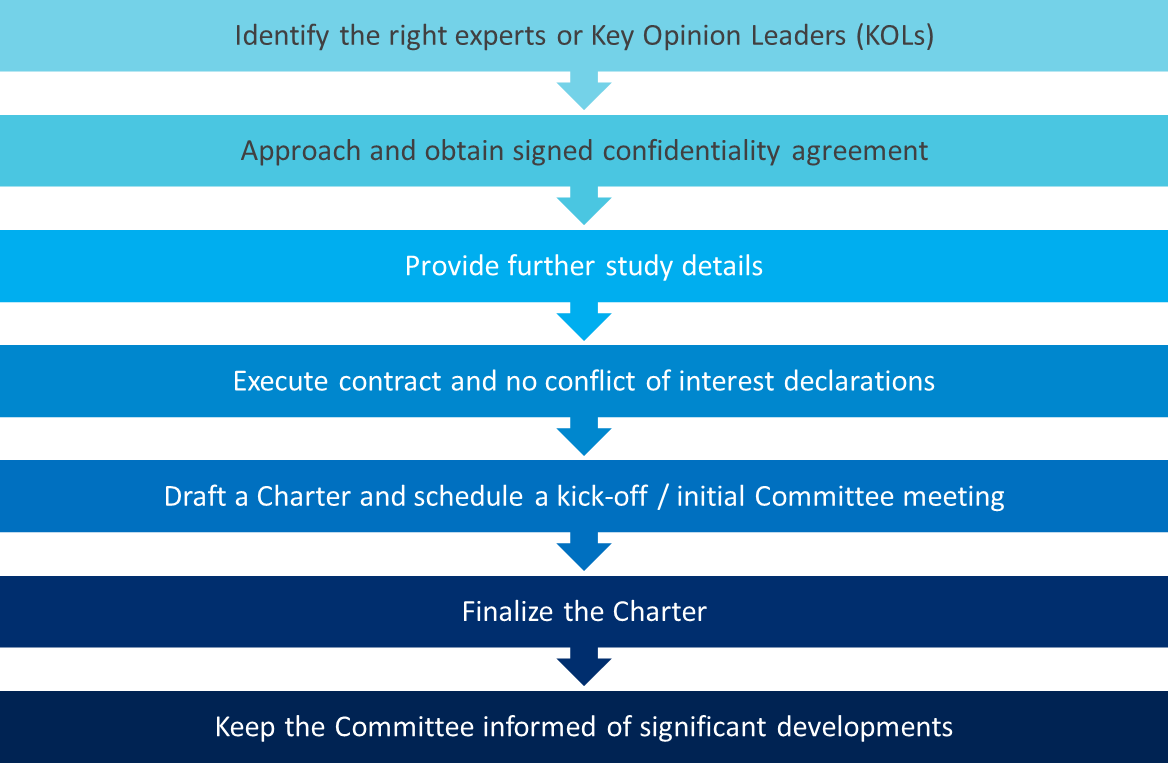
Sponsors should keep in mind that DMCs and CECs are not mutually exclusive. Depending on trial objectives, a study may call upon either committee or both. If both committees are involved, the CEC typically comes first and provides adjudicated data to the DMC.
Still not sure which, if any, committee is right for your medical device trial? Download our white paper, Decoding the Distinction: DMCs, CECs, and Their Role in Medical Device Research.
