Cell & Gene Therapy Expertise
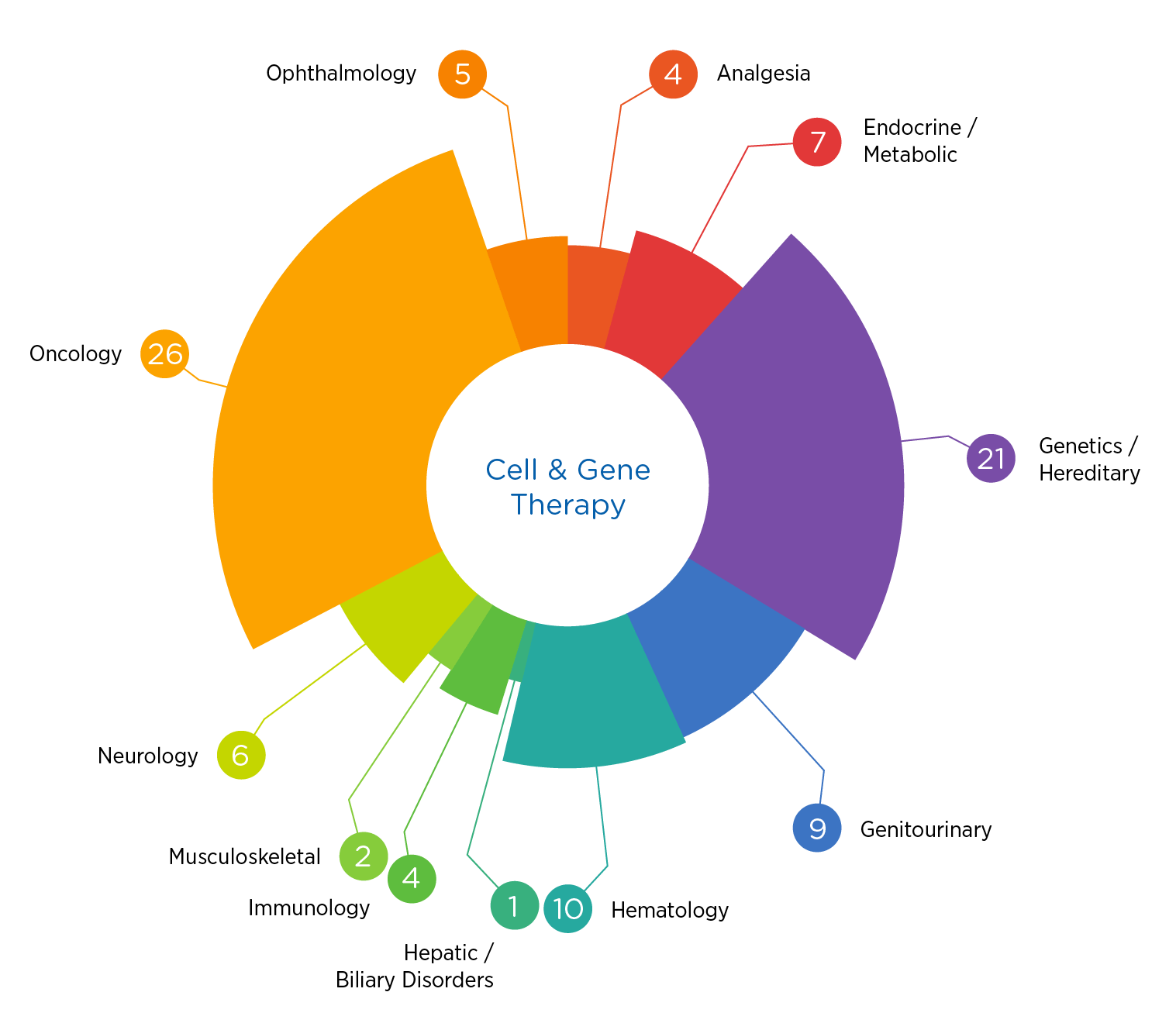
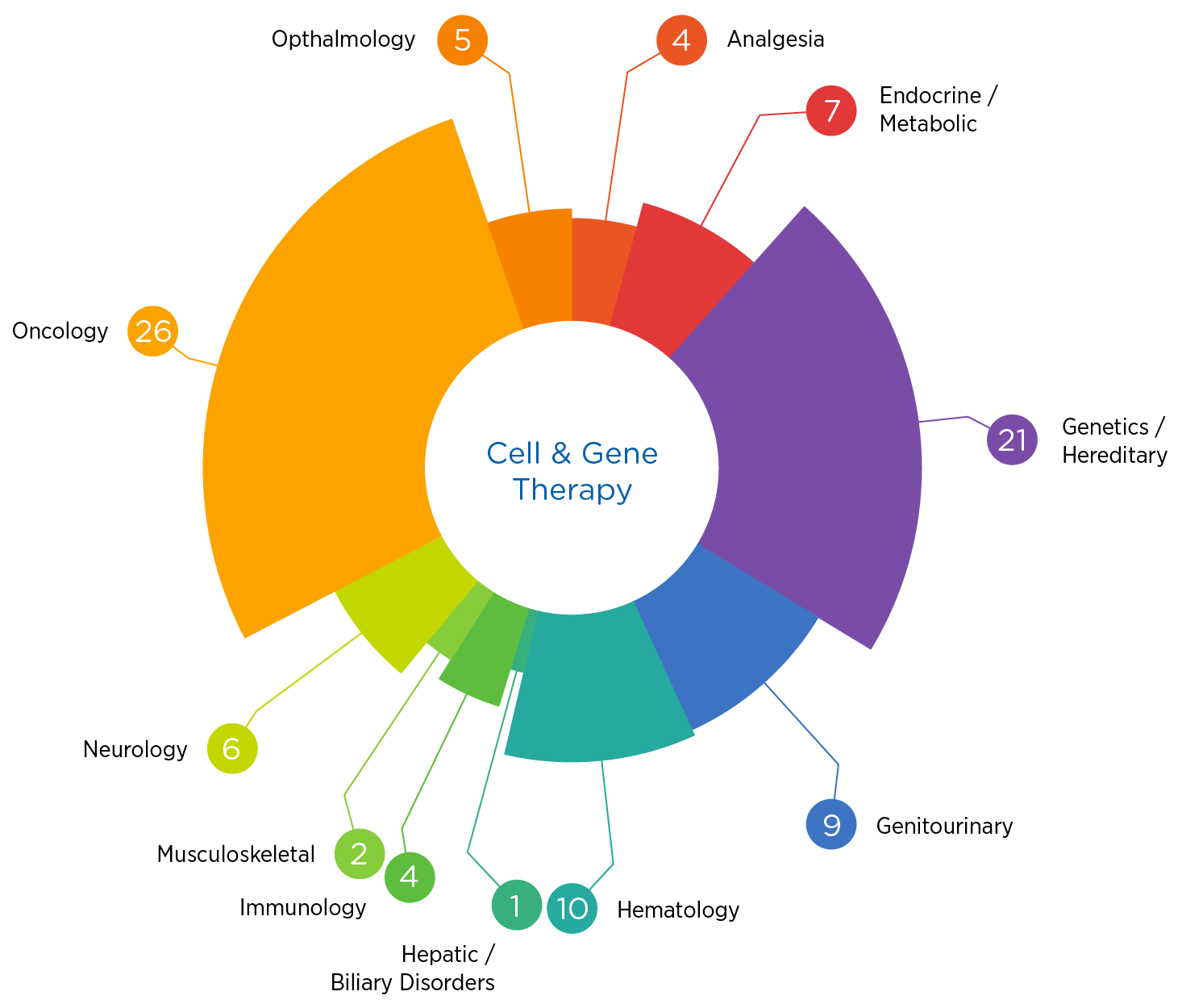
The science is stunningly complex, and the regulatory terrain is constantly evolving. Having supported over 90 cell and gene therapy trials in the past five years spanning oncology, hematology, metabolic disorders, and more, we know what it takes to succeed.
Supporting the evolving science of cell and gene therapy
Even measured against the vast scientific mystery that defines the biotech industry, cell and gene therapy poses extraordinary challenges. To achieve operational excellence in these trials, you must understand — and overcome — obstacles ranging from start-up regulations and site selection to patient recruitment and retention. And given the limited data on the long-term effects of these therapies, participants in cell and gene therapy trials may be monitored for a long-term follow-up period, which may be as long as 15 years.
For projects of this scale, you need a partner that can deliver from start to finish. That’s where Premier Research comes in.
We combine deep therapeutic expertise in oncology, rare disease, and pediatric research with other key areas, such as collaborative monitoring processes and technology. Together these allow us to deliver high-quality outcomes and an unwavering commitment to developers of next-generation therapies that makes us truly Built for Biotech℠.
Why choose Premier?
- Over 90 cell and gene therapy studies conducted in the past five years
- Significant experience in viral vectors
- Significant experience in both oncology and non-oncology
Cell and Gene Therapy
Premier’s end-to-end capabilities in complex asset development transport breakthrough therapies through an evolving clinical and regulatory landscape to deliver novel treatments to patients in need. Get our brochure.
Managing long-term follow-up studies
To understand and mitigate the risk of delayed adverse events, participants in gene and cell therapy trials may be monitored for a long-term follow-up period. Learn what’s needed and how to determine if your product requires LTFU studies.
Featured Resources
Premier Insight
#272: Delivering the Global Expertise Required to Coordinate a Pediatric Gene Therapy Trial
White Paper
Operationalizing Gene Therapy Trials
Premier Insight
#274: Site Selection, Manufacturing, & Long-Term Follow-Up in a Transition Phase 1/2 Gene Therapy Trial
Connect with us
Ready to get started? So are we. Drop us a line to learn more about how we can help.