Minimizing the burden that trial participants experience is essential for successful recruitment and retention, especially in oncology studies, where patients may need to be followed for years after the treatment phase of the trial. For sponsors, the process of optimizing study outcomes involves a deep understanding of the patient’s care journey and a deliberate effort to incorporate patient-gathered insights into trial design.
In this blog post, we explore protocol development considerations to reduce patient burden and enhance the overall clinical trial experience.
Streamlining Clinical Trial Assessments
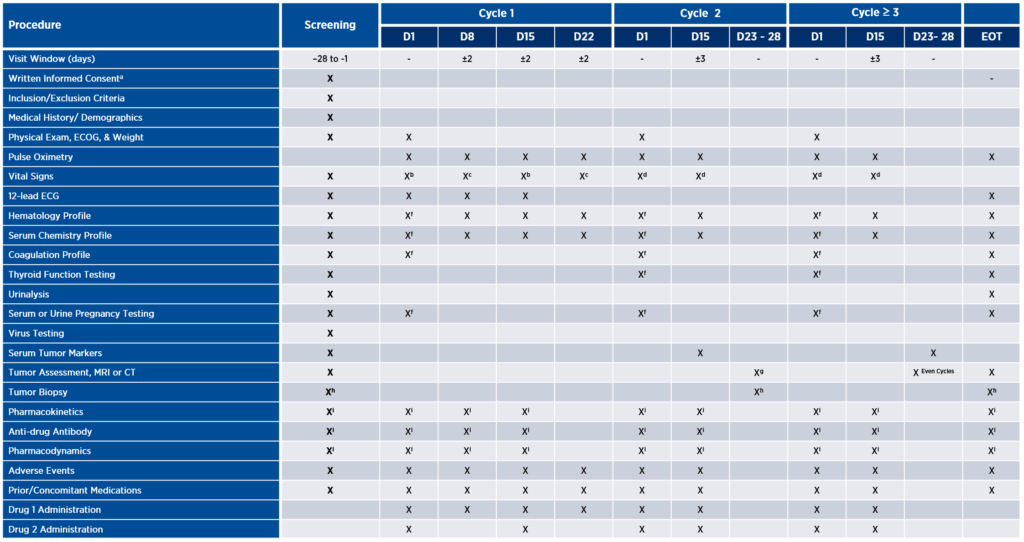
While assessment schedules vary from study to study, early phase oncology trials often involve weekly visits, serial blood draws, and multiple biopsies (see Figure 1a). Each of these assessments may be deemed critically important for ensuring the safety of patients and the completeness of the data collected. However, sponsors must keep in mind that patients will be taking on these extra burdens beyond the standard of care (SOC) (see Figure 1b). Although it may not be possible to reduce the number of clinical trial assessments to be on par with SOC, it is possible to take a critical look at the number, frequency, and location of assessments with a view toward reducing burden wherever possible without sacrificing patient safety or data quality.
Figure 1. Illustrative comparison of clinical trial assessments and standard of care
A. Clinical trial assessments
B. Standard of care
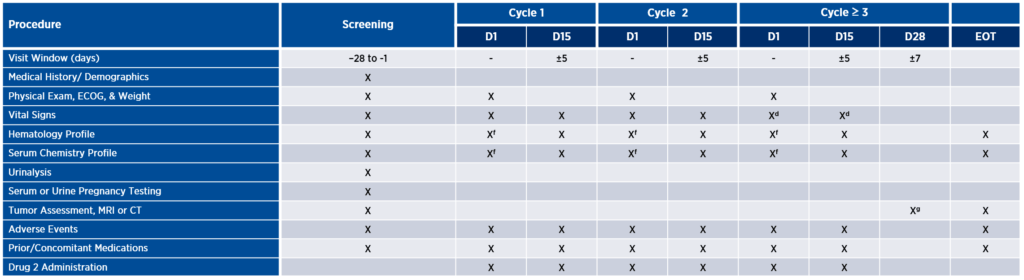
Burden can be defined in many ways, from impact on daily activities and invasiveness of procedures to effects on physical, emotional, or financial well-being. Consider, for example, tumor assessment via imaging as depicted in the table above. This study design calls for patients to be assessed for response every eight weeks with MRI or CT. Depending on the tumor indication and the patient’s insurance coverage, imaging might only be covered every 12 weeks. In such cases, the patient may be charged for these scans if they are not covered by the sponsor. To alleviate burden, the sponsor might consider whether there is any available preclinical data to support response assessments every 12 weeks, instead of eight. If not, the next step might be to implement a plan upfront to cover those additional expenses, thus reducing the patient’s financial burden.
This hypothetical study design also requires serial blood draws for pharmacokinetic (PK) and pharmacodynamic (PD) assessments up to 12 hours post-dose, resulting in a very long day for the patient. Rather than designing protocols based on what will provide the most complete data package, sponsors may benefit from shifting their lens to the degree of participant burden associated with each assessment and carefully consider whether it may be possible to achieve successful study outcomes with a smaller patient commitment.
Integrating Remote Assessments
If an assessment is determined to be critical, there may be ways to make the evaluation less burdensome to the patient than traditional study designs allow for. In this hypothetical study, the participant is expected to come in on days two and three for blood draws. Assuming both assessments are essential, sponsors may want to consider contracting with a local lab closer to the patient’s home or a home health vendor. This would allow participants to spend more time in the comfort of their own homes, rather than at or near a research site.
Incorporating remote assessments into a protocol requires sponsors to review all planned assessments and to determine which must be performed on-site under the supervision of the study principal investigator and which may be performed off-site. In early-phase oncology studies, the bulk of the assessments may need to be done on-site to ensure patient safety. In later-phase trials, where the safety profile is better known, more can be done remotely, but only if the right vendors are in place to facilitate sample and data collection.
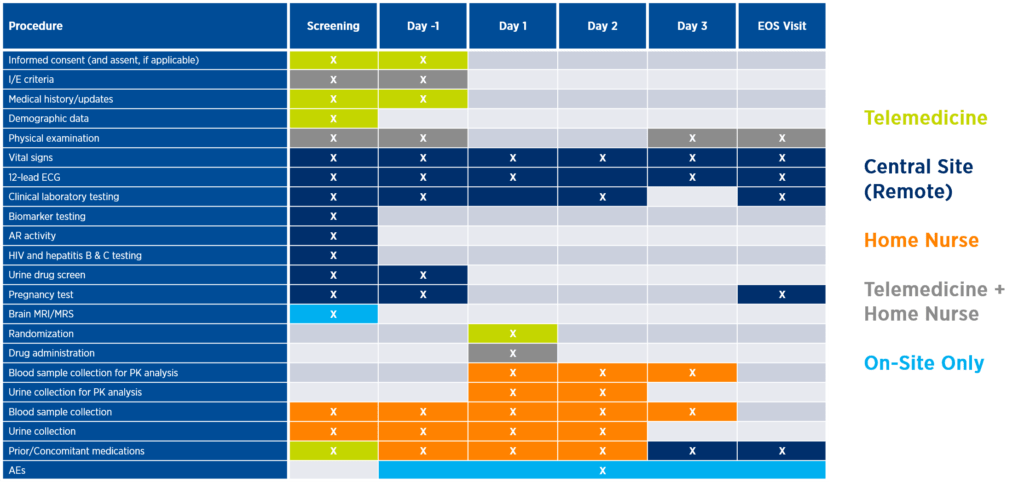
Sponsors may be surprised how many assessments can be performed via telemedicine, home nursing, or a combination of both, with appropriate upfront planning (see Figure 2). Incorporating community or central sites can not only reduce the need for travel but also increase the diversity of the trial population. These considerations will be unique to each study, but all lie on the continuum of decentralization and help sponsors bring trials closer to patients. The future of many late-phase oncology trials may be one central site with remote patients around the globe whose data is centrally monitored in real time. A prerequisite to the success of this fully decentralized model is having a well-thought-out strategy for recruitment, retention, and long-term patient engagement.
Figure 2. Illustrative incorporation of remote assessments into the study protocol
Creating Positive Patient Experiences
During the protocol development phase, sponsors may benefit from considering services and technologies that enable decentralization and enhance the clinical trial experience. Concierge services are available to provide end-to-end travel planning for patients and their families, from accommodations to reimbursements. Having an expert handle these logistics reduces travel interruptions and increases retention.
Offsite research nurses are another valuable resource. Service offerings vary by vendor, but may include:
- Collecting, processing, and shipping biospecimen samples
- Receiving IP shipments and performing IP accountability
- Administering IP
- Administering assessments or questionnaires
- Obtaining consent
- Performing study procedures such as physical examinations or electrocardiograms
- Collecting data on adverse events or concomitant medications
Meeting patients where they—and their families—are, and where they are most comfortable, is a powerful way to foster positive experiences that keep patients engaged, not just for a single trial, but for future trials as well.
Telemedicine enables remote visits via HIPAA-compliant video conferencing, while protecting patient data and providing an audit trial. The pandemic has increased acceptance and adoption of this modality and other technologies. Wearables with sensors that collect data which can be transmitted and integrated directly into the electronic data capture (EDC) system are beneficial for both patients and sites. Apps that collect patient-entered data or provide patient education are becoming more common, as are electronic patient reported outcomes (ePRO) and eConsent.
Constructing a Digital Patient Journey
Integrating and implementing remote solutions enables sponsors to construct digital patient journeys that reduce patient burden, increase engagement, and allow for instant access to data through continuous remote monitoring (see Figure 3).
Figure 3. Illustrative digital patient journey
Appropriate remote solutions will vary by study, but by viewing a protocol through the eyes of participants and their families, sponsors can design trials that create positive patient experiences with worthwhile downstream impacts on recruitment, retention, and ongoing engagement. For a deeper dive into this topic, view our webinar titled “Empowering Oncology Patients to Maximize Study Outcomes” here.
