During the initial planning for clinical trial implementation, intense focus is placed on strategies for patient recruitment, while strategies for patient engagement and retention are often left until after the treatment phase. However, with many current protocol designs in oncology, trial endpoints require study participants to be followed for years to evaluate side effects and obtain long-term survival rates. Patients who are not adequately engaged are unlikely to complete this long-term follow-up, even if they manage to stay throughout the treatment phase of the trial.
Oftentimes, protocol amendments may be needed to change assessments to increase retention. Having to replace patients adds cost, delays study timelines, and may even adversely impact submission goals due to inadequate data. Moreover, patients who drop out early or fail to complete the study may view clinical trial participation as a negative experience, thereby reducing their likelihood of participating in future clinical trials.
Developing and implementing strategies for both recruitment and retention at the beginning of a trial can help to optimize study outcomes. In this blog post, we offer strategies for sponsors to consider during the study planning process to maximize patient engagement and retention.
Gathering patient insights
Patient engagement can be used, not just to provide protocol-specific insights and capture the patient voice, but also to create a framework for the overall recruitment and retention strategy. While data will always be critical to decision-making about the recruitment process, developing a deep understanding of the patient perspective layers in a more personal perspective. This is crucial in oncology studies, as patients have a broad range of experience with their diseases and their own ways of coping with their conditions, both physically and mentally. Understanding these nuances is key when presenting a clinical trial to a potential patient as a viable option.
Being intentional about gathering insights—from disease impact and current treatment, to unmet needs and barriers, to adherence—is critical for understanding patients and where they will be in their care journeys at the point of protocol enrollment. The insight-gathering effort should include not just the patients but also their family members and caregivers. Patient advocates and advocacy groups are also useful sources of information. Methodologies for gathering these insights include surveys, focus groups, roundtables, social media, email, and direct mail.
Supporting recruitment
Patients with cancer have unique relationships with their oncologists and, sometimes, even their entire care teams. Despite these strong connections, it is rare for patients with cancer to consent to participating in a clinical trial the first time the opportunity is presented to them. Instead, consent typically requires thoughtful discussions among the patients, their caregivers, the investigator, and even the referring oncologists. Providing sites with engagement materials that will support them in these discussions is vital.
Identifying site-specific time points or procedures where eligible study participants can be identified is also crucial. Walking through touchpoint scenarios with sites will generally uncover potential points of contact that may have been overlooked due to the sheer number of physicians and departments involved in a patient’s care. If patients are expected to be referred to a study by other physicians, identifying that referral pathway and ensuring that the patient feels supported and comfortable through the provider transition is key.
Another priority for recruitment is diversity, which is critical for developing therapies that are effective for all populations. Given the multifactorial barriers to clinical trial participation among minorities, increasing diversity requires collaboration among sponsors, sites, and communities.
Considerations for increasing recruitment diversity
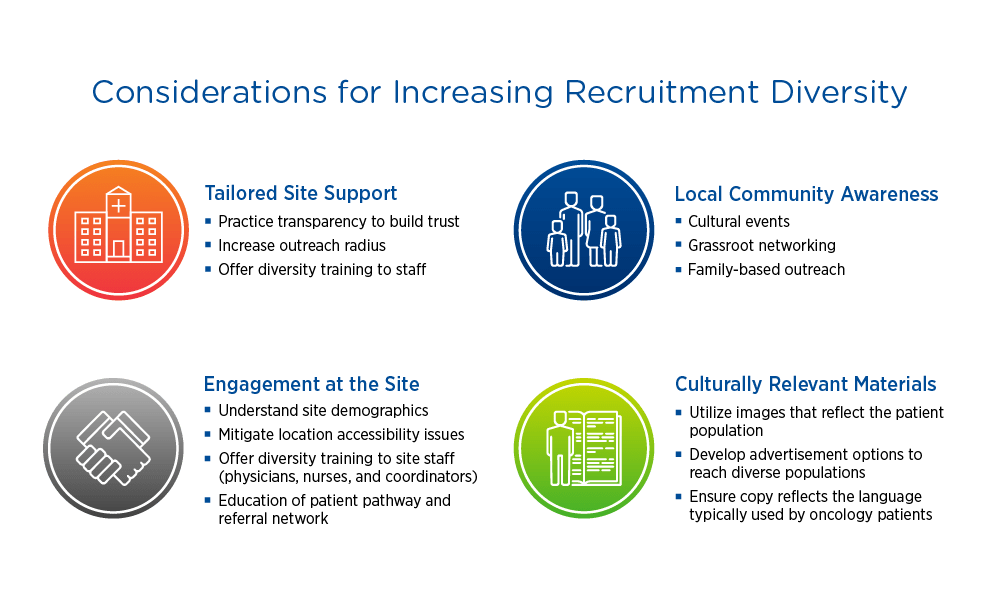
When evaluating sites, sponsors should consider demographics, reach, and staff diversity. Having a diverse staff that can speak to potential study participants in their language and understand their culture can make a big difference. Ensuring that recruitment materials are translated appropriately and include imaging that reflects the target population promotes informed consent and inclusion.
Mitigating risk
Risk is introduced any time a patient feels unsatisfied or worried about any aspect of clinical trial participation. Proactively identifying and addressing risks can mitigate potential enrollment and retention deficits. For example, providing free valet parking can ease the burden of walking a long distance from the parking lot to the study site.
Sample risk mitigation strategies
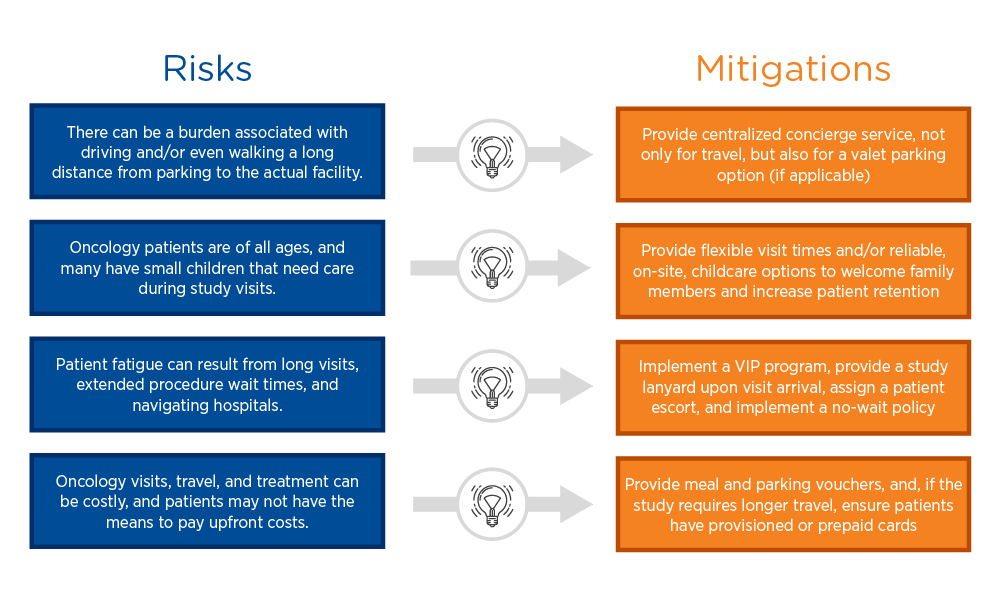
VIP programs can be extremely effective risk mitigation strategies. Oncology patients are accustomed to undergoing multiple tests and procedures, often with long wait times, which can be overwhelming. Implementing a VIP program that recognizes study participants with a lanyard allowing them to be first in line for a procedure, when possible, helps empower patients and ease the stress of a study visit. Coupling this with a printed schedule and an escort who can help them navigate to their next destination can transform the study experience for a patient.
Mitigating travel costs—including gas, parking, food, and accommodation, where relevant—is also important, as patients may not have the means to pay for these expenses upfront. Many studies offer travel reimbursement, but the most effective risk mitigation strategy is to eliminate out-of-pocket costs altogether by providing parking and meal vouchers or even provisioned or prepaid cards.
Maximizing retention
Research has shown that fear and anxiety during oncology clinical trial participation is the primary driver of early discontinuation. Consequently, the most successful retention programs focus not just on reminders but also on ongoing patient education. Educating patients on the importance of their commitment and what to expect at each visit can help to alleviate anxiety. They key is to anticipate patient questions and concerns and proactively provide thoughtful content that addresses them. Strategies to consider include sharing videos on how to take a study medication or what a procedure will involve and providing an electronic version of a study schedule, including specific instructions and expectations.
Communication and data sharing can be powerful strategies for ensuring a positive patient experience. Patients want to know what is being done with their data, and they want to know they are part of something bigger than themselves. Providing study participants access to their trial data empowers them and moves them from passive to active participants in their own health care. Importantly, certain digital data can be shared safely with participants while a trial is ongoing, rather than at the end of the study.
Data sharing is effective even when patients do not directly benefit from the clinical trial. For example, sharing response rates helps participants understand not only the significance of a trial for future patients but also their personal contributions to advancing cancer research. The St. Jude’s Childhood Cancer Survivor Study, which involves a retrospective cohort of over 38,000 pediatric cancer survivors, exemplifies how data sharing can create lasting engagement. These survivors have been providing feedback data about their health on a quarterly basis for over 10 years, and the study publishes a quarterly newsletter keeping participants up to date on information that is relevant to them.
Establishing patient communities
Optimizing engagement requires creating a community and a mechanism for patient feedback in real time; however, sponsors may lack the time or expertise to put these in place on their own. Social media platforms where patients and caregivers candidly discuss their unmet needs provide an opportunity to tap into unbiased viewpoints. Partnerships with advocacy groups can provide additional insights. Patient engagement specialists or CROs with experience in this area can also serve as the conduit between sponsors and patients. These specialists can help patients as they navigate available clinical trial information, obtain feedback on patient needs, and then share this information with sponsors, creating a timely communication loop that ensures patient voices are heard.
Key takeaway
Implementing patient-focused strategies from the initial stages of study planning increases enrollment, enhances retention, reduces protocol amendments, and, ultimately, leads to more complete data. Equally important, leveraging patient insights in protocol design helps create positive patient experiences and increases awareness and the likelihood of participating in future trials.
For a deeper dive into this topic, view our webinar titled “Empowering Oncology Patients to Maximize Study Outcomes.”
