Healthcare payers, regulators, and physicians focus on clinical utility when evaluating molecular diagnostics tests. Clinical validation — establishing that the test can make a diagnosis or predict clinical outcomes — may not be enough to garner acceptance. This point is crucial, because failure to gain acceptance deprives patients of much-needed health benefits and results in business failure for the developer. As a developer, to ensure adoption of a novel, precision medicine-enabling diagnostic test, you must demonstrate clinical utility — a process that can be challenging.
This blog series covers ways to approach designing and conducting a randomized clinical trial (RCT) to demonstrate clinical utility.
1. Focus on linking test results with healthcare outcomes early
Early on, diagnostics developers tend to focus on analytic and clinical validation rather than clinical utility. However, development plans should lay the groundwork for demonstrating clinical utility from the beginning. Satisfying regulators, payers, and physicians may require evidence gathered via one or more RCTs.
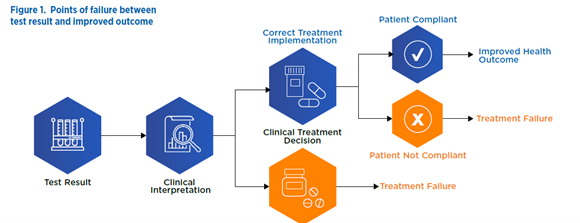
The success of clinical utility trials hinges on the complex sequence of actions that transform a test result into an improved health outcome. Every step between test result and health outcome may become a point of failure for the clinical utility study and therefore merits meticulous attention. Early attention to these transitions will help you develop the most cost-effective and efficient strategy possible for demonstrating clinical utility.
Points of potential failure between test result and improved outcome
2. Know your molecular diagnostic’s intended use, proposed indication, and target patient population
The context and specific requirements for demonstrating clinical utility will hinge on your diagnostic’s intended use and patient population. In addition, a risk assessment based on intended use and patient population determines the Food and Drug Administration regulatory pathway.
As the developer, you may need to submit a 510(k) Premarket Notification or Premarket Approval (PMA) application that “contains sufficient valid scientific evidence to assure that the device is safe and effective for its intended use(s).”
- A 510(k) or de novo submission may require a prospective clinical trial that accounts for risks associated with treatment decisions — based on false negatives or false positives — and medical procedures that may be ordered as a result.
- A PMA submission will likely require conduct of a well-controlled prospective clinical trial that demonstrates the diagnostic is safe and effective for its intended use.
3. Learn what influences physicians to make treatment decisions and which treatments are likely to result in improved patient outcomes
Depending upon the indication, a physician may choose from various treatments. The drivers for this decision may range from diagnostic results and safety and efficacy data to physician experience, judgment, and preference. Use knowledge of the various treatment options – and their pros and cons – to guide study design and operational planning to demonstrate the link between diagnostic result, treatment decision, and improved outcome.
4. Look for evidence that links treatment decisions directly to clinical outcomes
It may be possible to demonstrate clinical utility through a randomized study that shows that using the new diagnostic increases the likelihood that a physician orders a treatment already known to improve outcomes from previous RCTs. When such evidence exists, it may not be necessary to conduct a prospective RCT collecting new patient outcomes data.
A trial designed to assess changes in physician decision-making would randomize physicians rather than patients. One arm would receive the new diagnostic results (active), and the other would not (control). The study would gather evidence on whether the physicians in the active arm were more likely to make treatment decisions linked to improved outcomes.
5. Identify surrogate endpoints for the desired clinical outcome
Sometimes it’s not possible to link the results of an investigational diagnostic to an improved clinical outcome. However, you may be able to use an accepted surrogate endpoint for that outcome to reduce RCT costs and demonstrate clinical utility faster. You may still need to collect data to confirm the outcomes predicted by the surrogate endpoint, but this approach can enable earlier regulatory filing and accelerate the new diagnostic on its path to market. The surrogate data may also provide a basis for reimbursement by healthcare payers.
For example, a diagnostic test to evaluate the risk of coronary artery disease (CAD) could utilize the presence of low-density lipoprotein cholesterol (LDL-C) as one surrogate endpoint to support a CAD claim as the correlation between the two has been well established and would prevent significant effort in following subjects through to CAD events.
Reduce risk and save time with an efficient and effective clinical utility RCT
Diagnostics developers face unique challenges, and successfully demonstrating the clinical utility of a new molecular diagnostic is one of them. It requires the design and conduct of studies not only in diagnostics but also in therapeutics. Premier Research has extensive experience in both.
We have conducted numerous studies demonstrating the clinical utility of molecular diagnostics, supporting several successful 510(k) and PMA submissions. You can count on our in-house clinical and biostatistical experts to help plan, design, and execute a successful clinical utility program.
Contact Premier Research for customized services and efficient and immediate methods for demonstrating the clinical utility of your new molecular diagnostic.
