Diagnostic Development Expertise
Advanced diagnostics lead to improved treatment decisions—and improved patient health. Count on our expertise to help drive your development.
Developing diagnostics to support treatment decisions across the therapeutic spectrum
A sensitive and specific diagnostic can pinpoint a disease or monitor the efficacy of a line of treatment. Yet diagnostics developers face unique development challenges, from ever-evolving regulatory pathways to reimbursement hurdles. We can help. With a robust team of consulting experts, supported by full-service clinical development, we guide you through these challenges.
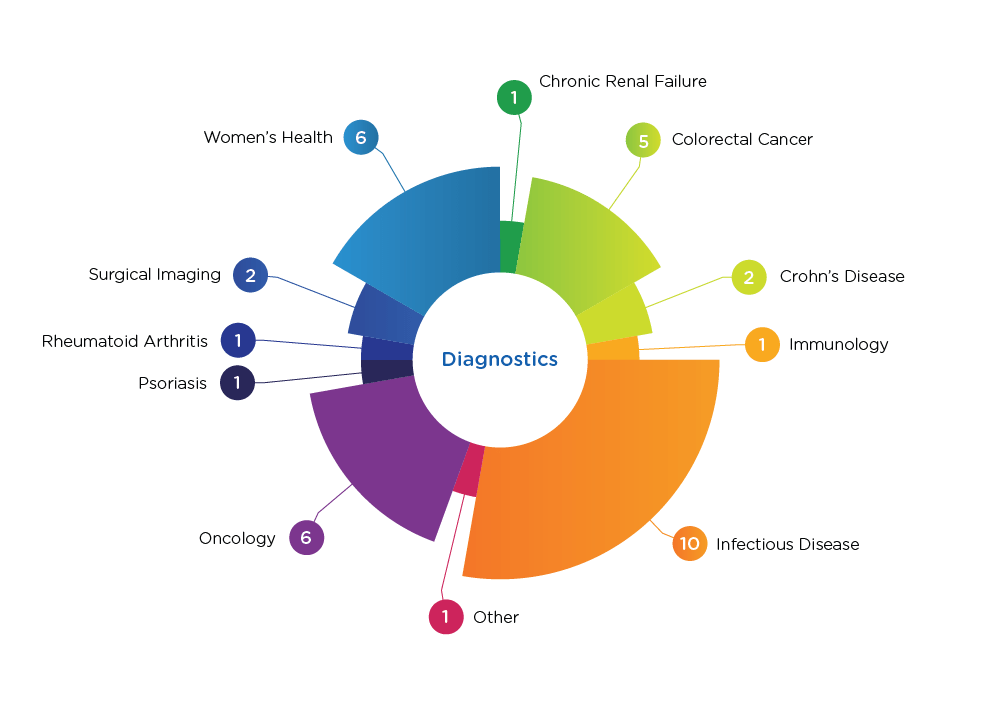
We’ve conducted successful studies for in vitro diagnostics (IVDs) and lab-developed tests—including point-of-care diagnostics, molecular diagnostics, immunoassays, and sample collection devices. While we work across various therapeutic areas, we provide specific expertise in oncology, infectious disease, women’s health, and gastrointestinal disease. In fact, our diagnostics franchise managed the first successful premarket approval of a colorectal cancer diagnostic that led to the only FDA-approved stool-DNA noninvasive colorectal cancer screening test.
- Customized services, scalable solutions. We work on diagnostic trials of all kinds—regardless of size, stage, setting, or data type—with clients of every size, serving as an extension of your development team. We approach each project with flexibility, then tailor solutions that help accelerate your development.
- Experienced clinical staff. From the project managers and clinical research associates who staff your study to the KOLs throughout our site network, you will partner with medical and procedural experts who can provide insights and help eliminate roadblocks.
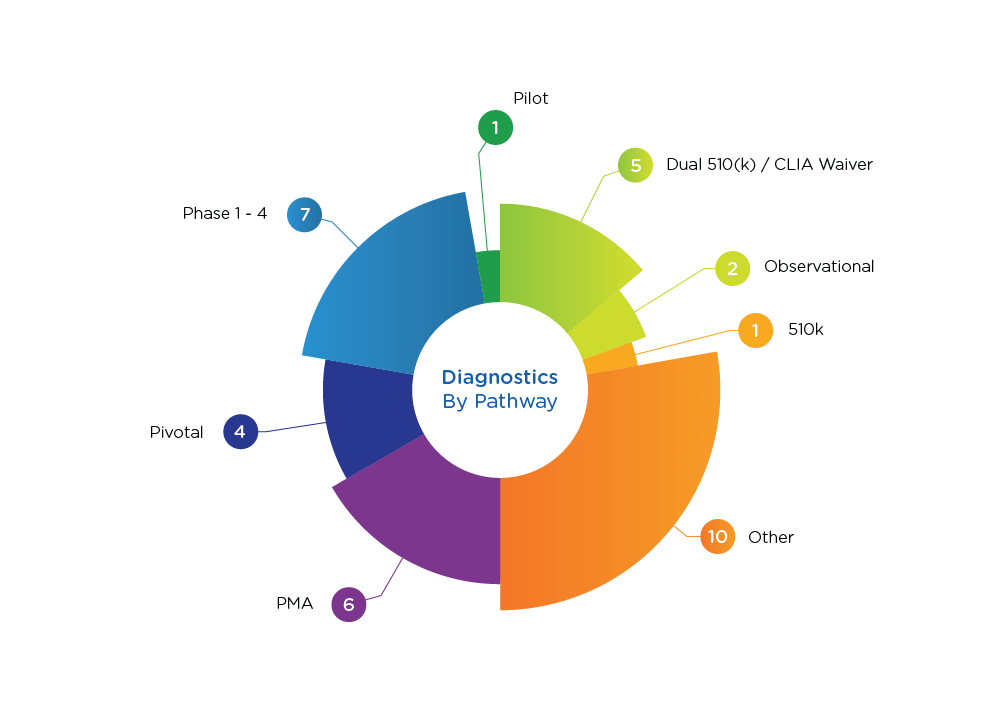
- Proven track record. With proven expertise in all manner of studies— PMA; 510k equivalence, de novo, point of care; and dual 510k/CLIA submissions—we are prepared to answer your FDA-related questions, review and support submissions, and analyze your protocols, your competitors, and your data verification. In short, we are ready to be your partner.
- Demonstrated speed to start-up. With a global network of sites with which we regularly partner, we can often waive the pre-study visit, accelerating timelines and lowering costs.
Therapeutic Area Expertise
- Oncology
- Infectious Disease
- Women’s Health
- Gastroenterology/Hepatology
- Pulmonology
- Analgesia
- Dermatology
- Transplant
SERVICE OFFERINGS
Full-Service Trial Management
- Study design
- Regulatory strategy
- Study execution
- Submission prep
- Submission filing
All Development Stages
- Sample collection
- Analytic validation
- Human factors/usability
- Reproducibility and repeatability
- Clinical validation
- Clinical utility
Effective Sample Management
- Prospective or retrospective collection
- Effective sample tracking
- Wide variety of specimen types
Range of Study Settings
- Labs
- Point-of-care facilities
- Phase 1 centers
- At-Home/Simulated Home
- Mobile facilities
Expansive Data Types
- Lab results
- CRFs
- Usability questionnaires
- QOL surveys
- Diaries
- Visual analog scales
Regulatory Pathway
- CE-marking
- IVDR
- 510(k)
- PMA
- LDT
- Dual 510(k)/CLIA
Diagnostics Indications
Diagnostics Pathways
PREMIER INSIGHT
Harnessing Critical Experience to Plan and Execute a Dual-Submission Study
Point-of-care (POC) tests offer significant convenience for patients and physicians, as well as cost-savings for payers. Yet, achieving regulatory approval can be daunting. Assembling such proof requires thoughtful trial design and data collection.
PREMIER INSIGHT
Engineering a PMA Study for a First-of-its-kind Oncology Dx
Colorectal cancer (CRC) is the second most deadly form of cancer – although it is among the most curable and the easiest to detect in its early stages. Our client developed a unique solution – a multi-target, noninvasive screening test that could be facilitated by patients in their homes. They came to Premier Research to conduct the pre-market approval (PMA) study.
Related Capabilities
A great strategy is nothing without great execution. Look here for experience in clinical R&D, strategic product development, and technology applications that ensure data integrity, process efficiency, and timely analytics and reporting.
Clinical Research & Development
Strategic Product Development

Premier Ecosystem

Check out our resource center
Our experts have developed an extensive library of white papers, case studies, blogposts, and other informative resources.
PREMIER INSIGHTS
WHITE PAPERS
WEBINARS
VIDEOS
PODCASTS
Connect with us
Ready to get started? So are we. Drop us a line to learn more about how we can help.