The challenge of enrollment for observational research
Patient enrollment and retention for investigational trials are notoriously difficult. How is enrollment affected when there is no direct benefit to the patient: no breakthrough study drug with the potential to cure, no added caregiver time, no meaningful compensation for participating?
Observational studies, by design, demand a continuation of the same standard of care a patient would have received without joining the study. Incentivizing these patients to participate is a significant challenge for sponsors engaged in post-marketing or other observational studies.
The lack of benefits, combined with the inability to use active recruitment tactics (e.g., advertising) or offer significant compensation, constitutes a fundamental challenge. Sponsors targeting a low-motivation indication, such as depression, find it even more difficult to recruit and retain the patients needed to complete an observational study. In this blog post, we outline some successful strategies developed to recruit for these studies and keep the patients engaged and retained.
For optimal recruitment, patients need a reason to believe
Even though they may not receive a tangible benefit for participating, patients do experience emotional benefits and, under the right circumstances, can be motivated to join and complete observational studies.
- Studies and our experience point to altruism as the top motivator
In our experience (and study after study1), altruism ranked highest among drivers for patients to participate in clinical studies. Next came intellectual and health-related reasoning, with financial motivations significantly lower in importance.
Some patients, such as those with hereditary diseases, have personal reasons for wanting to “give back” or help future generations. Finding a way to connect with potential study patients’ sense of the greater good is crucial.
- Each observational trial is different — observe what resonates and share that story among investigators
Patients’ motivations vary according to therapeutic area, how ill they are, gender, and more.2 Once sponsors understand special motivators that apply to their trial, they should share it, preferably in the form of patient stories, with trial investigators.
- Whatever the circumstances, recruitment for observational studies takes time
Observational study designs see slower enrollment than interventional designs, in general, for the reasons outlined above. Recruitment is not a matter of advertising, networking, or receiving referrals. Patients are found making routine visits at pre-selected investigator sites.
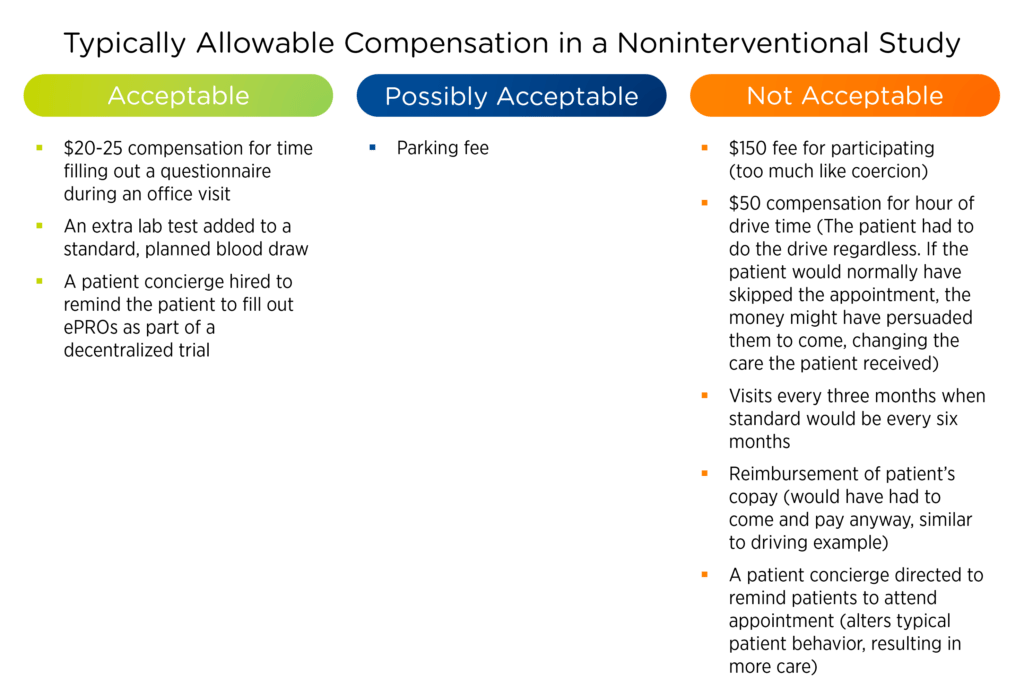
Allowable compensation for observational studies hinges on conformance with the standard of care
In observational, noninterventional studies, protocol objectives and assessments must reflect the treatment standard of care for the relevant patient population. According to the FDA, “A protocol-based investigation is noninterventional if the intervention of interest is given during routine clinical care, according to the clinician’s judgment.”3
Nothing in the protocol, such as compensation for additional activities, can cause caregivers or patients to deviate from what would typically be done. In reviewing the study protocol, IRBs may be quite strict in how they interpret this concept to ensure compensation does not change a patient’s behavior. The following examples help illustrate this idea.
Table 1
Select the right sites from the beginning to acquire and retain the patients you need
Late phase studies of long-term safety and efficacy are less time consuming than randomized clinical trials (RCTs) for investigators. However, for sponsors, they are fraught with patient recruitment and retention challenges. Recruitment success depends on leveraging provider relationships to select sites and investigators with enough qualified patients and the proper motivation to complete the study. Following these guidelines has proven highly beneficial:
- Seek out therapeutically experienced sites with sufficient resources and target patients
For these studies, based on routine clinical care, research experience is not critical. However, the site needs enough patients who fit the study criteria. Typically, sponsors have data on which hospitals, sites, and practitioners are already using the drug in question (assuming it’s a post-marketing study), and selection can start from there.
- Ensure the site is central enough to support the study
A remote site may have too small a patient base and may not support sufficiently frequent patient visits, even when called for by the standard of care.
- Gauge investigator motivation
Fees for observational studies are much lower than those for RCTs. Focus on other factors that may affect investigators’ motivation to complete observational studies:- Harness the desire to build sponsor relationships
Investigators may invest the time and effort of completing an observational study to bolster a new or existing sponsor relationship, prove themselves, and pave the way for future, more lucrative RCTs. - Check for competing projects
A coexisting RCT could pull an investigator’s resources away from an observational study. - Offer authorship if possible
Some investigators will be motivated by the chance to add articles to their CV.
- Harness the desire to build sponsor relationships
Help sites and patients to stay motivated and engaged
One key to success in observational studies is keeping both patients and sites engaged. A typical rule of thumb is that loss to follow-up should not exceed 20 percent of the sample.4 The below identifies proven strategies to maintain patient and site engagement.
- Patients
- Leverage provider-patient relationships
With little tangible benefit for participation, a trusted health care provider can deliver the information and encouragement patients need to stay involved in an observational study. - Utilize appropriate tools
Newsletters, brochures, educational resources, and patient advocacy groups can keep patients focused on the latest news related to their diagnosis and invested in future developments surrounding treatment. - Provide regular updates about the study
Knowing how the study is progressing helps patients see the impact of their involvement. - Keep the patient burden low
Decentralized study designs with evolving digital tools, such as ePRO, enable patients to stay home. Cellphone apps can simultaneously motivate patients and help investigators track adherence. Single points of contact such as patient concierges can help keep patients and their families organized and compliant. - Keep an open mind to determine what is effective
The best motivators may be indication- or protocol-specific. What’s worked in the past may or may not work now. Being able to extrapolate from one situation to another requires finesse.
- Leverage provider-patient relationships
- Sites
- Keep the site burden low
Limit data collection to the primary objectives. Implement labor-saving apps such as ePRO and eCOA systems to lighten the load for sites. - Leverage investigator meetings
Meetings allow for shared experience and can encourage competition, keeping investigators in the game.
- Keep the site burden low
Access tried-and-true strategies for successful late phase observational studies
Observational research presents very specific challenges. Sponsors must motivate patients and sites to participate in the absence of active recruitment tactics or the benefits that typically accompany RCTs. Sites must be selected strategically, and protocol objectives and assessments may not deviate from the routine standard of care.
The Premier Research real-world and late phase team has the tools and strategies necessary to overcome these challenges and rate-limiting factors to help sponsors succeed with their real-world science and late phase programs. Contact us to learn more about our full-service and standalone options utilizing cost-effective strategies for efficient observational studies.
[1] Soule MC, Beale EE, Suarez L, Beach SR, Mastromauro CA, Celano CM, et al. Understanding motivations to participate in an observational research study: Why do patients enroll? [Internet]. Social work in health care. U.S. National Library of Medicine; 2016 [cited 2022Apr14]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4870048/
[2] Goodman D, Johnson CO, Bowen D, Wenzel L, Edwards K. Factors that motivate participation in observational genetic cancer research studies [Internet]. Open Journal of Epidemiology. Scientific Research Publishing; 2019 [cited 2022Apr14]. Available from: https://www.scirp.org/journal/paperinformation.aspx?paperid=92676
[3] Concato J, Corrigan-Curay J, Ball R, Dal Pan GJ, Stein P. Randomized, observational, interventional, and real-world-What’s in a name? [Internet]. Pharmacoepidemiology and drug safety. U.S. National Library of Medicine; 2020 [cited 2022Apr14]. Available from: https://pubmed.ncbi.nlm.nih.gov/32940401/
[4] Song JW, Chung KC. Observational studies: Cohort and case-control studies [Internet]. Plastic and reconstructive surgery. U.S. National Library of Medicine; 2010 [cited 2022Apr14]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2998589/
