Adaptive design is a progressive approach that utilizes different statistical modeling to allow for modifications to enrollment in a clinical trial after study initiation without undermining data integrity and validity. This methodology is particularly beneficial and increasingly common in phase 1 clinical trials, which focus primarily on safety, tolerability, pharmacokinetics, and pharmacodynamics.
The essence of adaptive design
Adaptive design strategies involve a predefined plan that allows for adjustments based on interim analysis of collected data. These adjustments might include changes in sample size, dose adjustments, or even the cessation of the trial for efficacy or safety concerns. The primary objective of adaptive design is to make clinical trials more flexible, efficient, and informative, and to find a more accurate therapeutic dose for subsequent trials. Utilizing adaptive strategies often reduces costs, time, and resources required to bring new therapies to market.
The emergence of seamless phase 1/2 adaptive designs
Seamless phase 1/2 adaptive designs have emerged as a sophisticated strategy for combining traditionally distinct phases of drug development to streamline the evaluation of new therapies. In seamless phase 1/2 adaptive designs, dose escalation studies to assess safety and tolerability transition into efficacy studies without having to pause and restart the trial. The success of this approach is driven by predefined criteria—such as dose adjustments, changes to enrollment criteria, or selection of therapeutic indications—that guide modifications to the course of the study based on accumulating data.
9 ways seamless phase 1/2 adaptive designs can optimize efficiency
Seamless phase 1/2 adaptive designs:
- Minimize the need for amendments or new protocols, provided that a priori assumptions and study design are accepted by regulatory authorities.
- Allow for early efficacy read-out using a 2-stage design.
- Can include continued dose finding with combination regimens.
- Can continue treatment of any responders in phase 1 dose finding without the need for expanded access.
- Eliminate the time gap between different phases of a study.
- Allow for discontinuation of arms that show minimal effect in appropriately-sized dose expansion cohorts.
- Allow trials to continue with the same active sites, avoiding delays associated with study startup.
- Enable intrapatient dose escalation once safety is cleared in higher dose cohorts.
- Enhance decision-making through interim analyses that allow trial parameters to be optimized in real-time.
Example of a seamless phase 1/2 adaptive design
A well-designed seamless phase 1/2 trial can yield a wealth of data within the phase 1 setting.
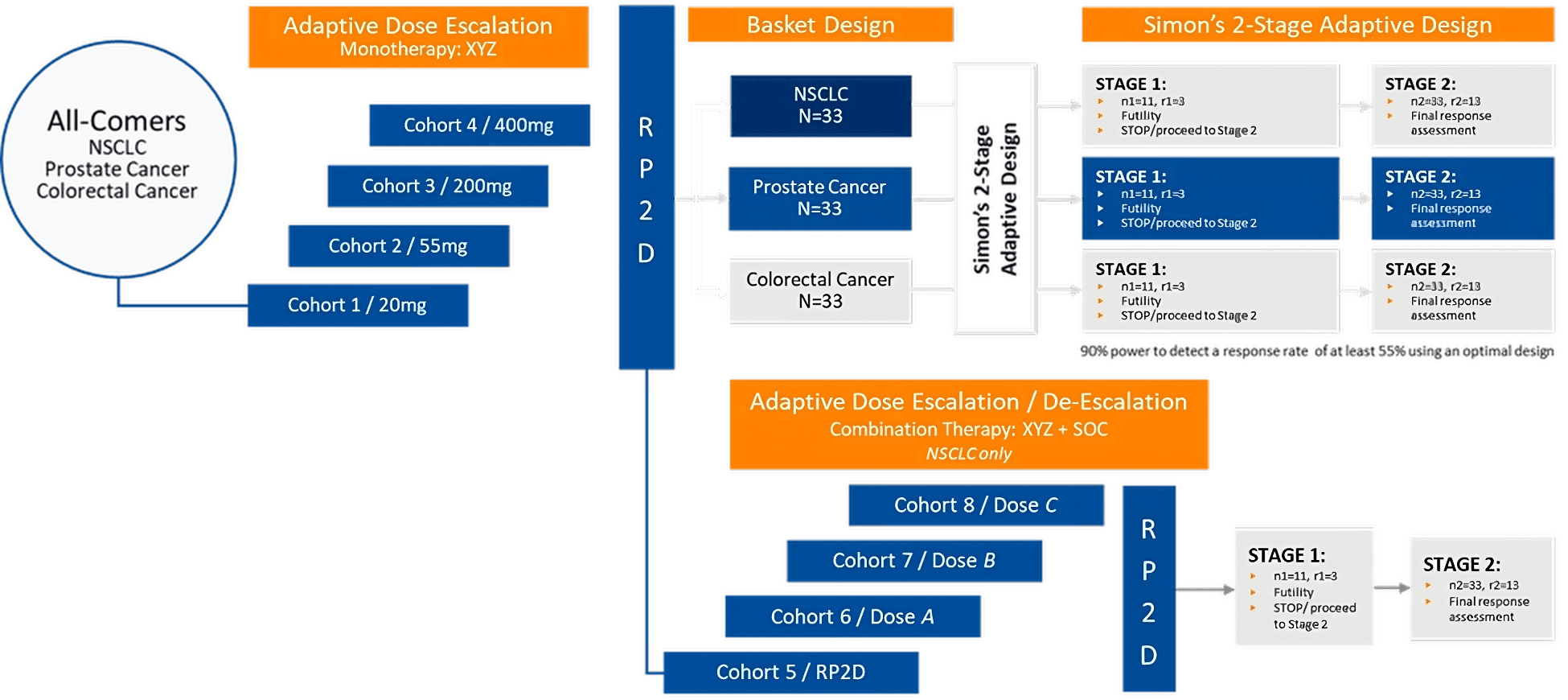
Figure 1. Example of a seamless phase 1/2 adaptive design
In the example above, a Bayesian Optimal INterval (BOIN) design was used for initial monotherapy dose escalation in a trial seeking to evaluate the effect of a new PD-1 inhibitor, XYZ, on tumor response, as either a monotherapy or in combination with standard of care in patients with non-small cell lung cancer (NSCLC), prostate cancer, and colorectal cancer.

Figure 2. Focus on phase 1 of the adaptive design
Phase 1 involved adaptive dose escalation of XYZ monotherapy at four doses. Bayesian prior probabilities of dose limiting toxicities (DLTs) were set for each dose level. Adaptive dose escalation was based on estimating the Bayesian posterior probability of observed DLT, with decision rules based on the value of posterior probabilities relative to the target DLT rate of 30%.

Figure 3. Focus on phase 2 of the adaptive design
Once a recommended phase 2 dose (RP2D) was identified, it was used:
- In a basket study involving three cohorts, one for each tumor type, which was guided by Simon’s 2-stage design. The advantage of this approach was that futility in one cohort would not stall the trial.
- In an adaptive dose escalation/de-escalation study of combination therapy in NSCLC only to identify an RP2D for another 2-stage design.
Key takeaway
Seamless phase 1/2 adaptive designs offer a promising pathway for enhancing the efficiency and efficacy of clinical trials. By integrating the exploratory and confirmatory phases of drug development, these designs can accelerate the evaluation of investigational therapies, optimize resource utilization, and improve the quality of clinical decision-making.
To learn more about mastering dose escalation studies, contact us.