Last Updated: October 22, 2024, 1 pm UTC
Post-market clinical follow-up (PMCF) activities are necessary for continuously monitoring the clinical benefits, performance, and safety of medical devices after they have been released on the market. As part of a broader post-market surveillance (PMS) strategy, these activities are crucial for assessing the long-term behavior of a device and ensuring that the risk-benefit profile remains favorable when the device is used as intended. PMCF activities are also required for regulatory compliance under the EU Medical Device Regulation (MDR).
Prior to the implementation of the EU MDR, the strict requirement for PMCF activities was limited to higher risk devices. Now, under MDR Annex XIV Part B, PMCF is required, regardless of risk classification, and should be a continuous process throughout the lifetime of a device. Though it may be possible to justify non-application of PMCF during clinical evaluation, most device developers will need to plan for PMCF activities.
In this blog, we dive into the regulatory framework for PMCF activities, highlighting the importance of developing a PMCF plan early in the development process.
Objectives of PMCF activities
PMCF is a systematic and proactive method of gathering clinical data on device use and outcomes. The goals of PMCF are to:1
- Confirm the safety and performance, including the clinical benefit(s), of a device throughout its expected lifetime
- Monitor known side effects and identify previously unknown side effects
- Identify and analyze emergent risks based on factual evidence
- Ensure continued acceptability of the benefit-risk ratio
- Identify possible systematic off-label use or misuse, whether intentional or accidental, of a device
Developing a PMCF plan
Developing a comprehensive PMCF plan as part of the overall PMS strategy early in the device lifecycle is key. Both the PMCF plan and the evaluation report of any PMCF activities count as clinical data. As such, these documents are part of the technical documentation of a device under MDR requirements.2 Advance planning helps maximize study outcomes and address multi-stakeholder requests or expectations.
A PMCF plan must describe activities or methods for gathering data on clinical performance and safety. It is not enough to simply document which activities will be conducted. It is also necessary to identify the objective of each activity, provide a scientific rationale for its appropriateness, and indicate a timeline and deadline within which that activity will be performed. The notified body (NB) must verify that the PMCF plan and its implementation are adequate. Further, the notified body must provide an assessment of consistency of the clinical evidence with the PMCF plan.3
Types of PMCF activities
PMCF activities fall into two broad categories—general and specific (see Figure 1).4
General PMCF activities are information-gathering activities that produce a subjective dataset. Typically, the datasets generated by general PMCF activities cannot stand alone as scientific documentation of clinical performance and safety.
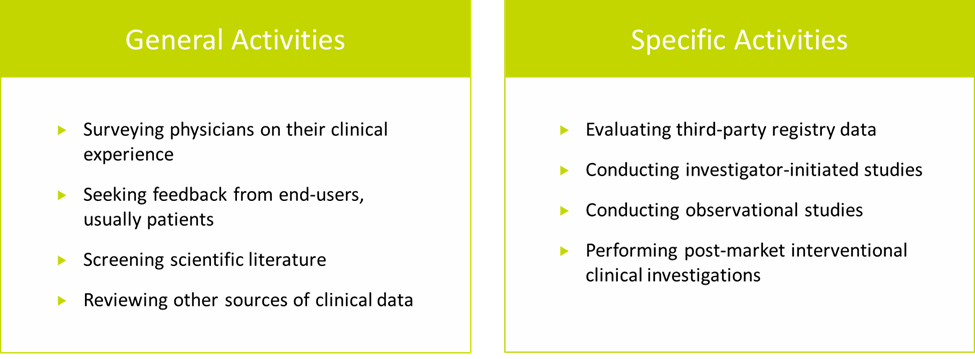
Figure 1. Examples of PMCF activities
Specific PMCF activities, on the other hand, are designed to produce an objective dataset that can be used to demonstrate clinical performance and safety. Specific PMCF activities include:
- Evaluating third-party registry data. Third-party registries are more common for high-risk (Class III) and long-term implantable devices. While registries often provide the level of detail and traceability necessary for PMCF, the type and quality of data collected can vary widely from registry to registry.
- Conducting investigator-initiated studies. These types of studies are becoming increasingly popular among device manufacturers for gathering clinical data, and they can be valuable sources of subject-specific data and clinical outcomes. However, as with registries, the type and quality of data collected can vary.
- Conducting observational studies, either on a single cohort or a series of patients. These require not only access to patients, but also collection of outcomes that are relevant to PMCF requirements.
- Performing post-market interventional clinical investigations is the most robust approach to collecting high quality data, but is the most resource-intensive and may require long-term follow-up.
An interventional PMCF clinical investigation may be necessary if any of the following conditions are met:
- CE mark was obtained under the Medical Device Directive (MDD) based on equivalence
- The device is based on novel technology
- The long-term safety or performance of the device is unknown or unclear
- The device is intended for a high-risk population, as is the case with implantable devices
- The device has not been studied in subpopulations that may have a different benefit-risk ratio
- The device has the potential to interact with other medical products or treatments
Selecting the right PMCF activities
Each type of PMCF activity has its pros and cons and the appropriateness of any activity should be evaluated in the unique context of a particular device. The methodology of PMCF activity should be based on its objective, as well as the type, risk profile, and duration of use of the device, and other factors that may impact its safety or performance in a clinical setting.
Factors to consider when selecting the most appropriate PMCF activity or activities for a device include:
- PMS reporting requirements may vary by device classification and reporting deadlines may be shorter for certain devices, limiting the time available to gather data for the first PMCF report
- Clinical claims of safety and performance may require additional substantiating clinical evidence
- Level of sales may influence PMCF activities because, if sales are low, it can be difficult to collect sufficient data from observational activities, or could strategically be used to increase sales
- Data available from earlier clinical evaluations will help inform the level of PMCF data
- Device performance measures and clinical/industry standards will also influence the type and level of data that needs to be collected from PMCF activities
- Access to customers, end-users, and patients is necessary for certain data collection activities, such as surveys
- Intended use of the device will impact who data can be gathered from
- Existence of a data consent will dictate whether it is possible to access data from patient registries or other databases under GDPR
Key takeaway
To develop a strong PMCF plan, begin with the end in mind. It is essential to be strategic about what data to collect. First, consider what results need to be presented to achieve critical objectives, including successful commercialization. Then, select the most appropriate activities to support those results. The level of clinical evidence generated should be proportionate and appropriate to the nature, classification, intended purpose, and risks of both the device and the manufacturer’s claims about the device.
Developing a PMCF plan as early as possible in a device program helps ensure efficient design and implementation of any PMCF activities, especially if clinical investigations are required.
To learn more about designing and conducting a successful PMCF clinical investigation, click here.
[1] MDR, Annex XIV, Part B, 6.1.
[2] Johner Institute. Post-Market Clinical Follow-up: Best Practices for PMCF, November 1, 2023. Available at https://www.johner-institute.com/articles/regulatory-affairs/post-market-clinical-follow-up-pmcf/.
[3] MDCG-2020-13.
[4] MDR, Annex XIV, Part B, 6.2.

 Webinar
Webinar 

 Perspectives Blog
Perspectives Blog 