Antibody-drug conjugates (ADCs) are an active area of oncology research, partly due to advances in synthetic biochemistry that may help improve the tissue specificity and cytotoxicity of these complex therapeutics. In recent years, the pace of development for this class of cancer therapeutics has been increasing, with 12 ADCs approved by the FDA since June 2019.
In this blog, we explore the current landscape of antibody-drug conjugates, including their benefits, limitations, key considerations for clinical trials, and potential future trends.
What is an antibody-drug conjugate?
An antibody-drug conjugate (ADC) is a therapeutic comprised of a monoclonal antibody (mAb) chemically attached to a drug or pro-drug, referred to as a payload, by a linker. The linker ensures that the cytotoxic payload remains attached to the mAb while the drug circulates in plasma. The mAb is designed to bind to a tumor-associated/tumor-specific antigen on the tumor cells and, after binding, the ADC is internalized by the tumor cells and the payload is released, causing cell death. While early ADCs were designed to carry traditional chemotherapeutics with known anticancer activity, currently approved ADCs carry more potent drugs as it has been shown that greater cytotoxicity is necessary for therapeutic efficacy.1
The primary objective of an ADC is to improve the therapeutic index of an antineoplastic drug by limiting its systemic delivery to cells expressing the target antigen of the selected mAb.2 The target antigen should be preferentially expressed in tumor versus non-malignant tissue to decrease the likelihood and magnitude of systemic toxicities.3
Benefits of ADCs
ADCs offer several benefits as potential cancer therapies:
- Antibody directed antigen/target specific delivery of cytotoxic drugs to tumor cells while sparing normal cells, improving tumor-to-normal tissue selectivity and specificity
- Internalization of the payload allows for increased drug potency coupled with limited systemic exposure, manifesting clinically as a wider therapeutic window and fewer side effects
- Certain ADCs exhibit activity in cancers that are resistant to cytotoxic agents with the same target or primary mechanism or action as the ADC2
Limitations of ADCs
While many of the currently approved ADCs demonstrate significant efficacy against treatment-refractory cancers, broader use of these therapies remains limited by systemic toxicity, lack of validated biomarkers for patient selection, poorly understood mechanisms of resistance, and absence of data on their use in combination with other therapeutic modalities. ADCs with cleavable linkers and payloads that can cross the cell membrane may have cytotoxic activity against antigen-negative cells that are in close proximity to antigen-positive cells via a bystander effect.2 Moreover, the mAbs in ADCs are large molecules and their size may limit their delivery to tumors and their ability to penetrate tumor tissue.
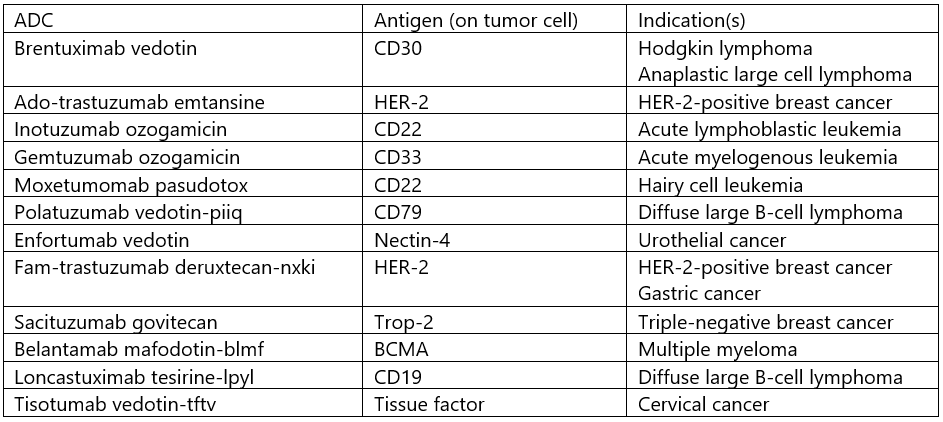
Approved ADCs
As of June 2022, the FDA has approved 12 ADC cancer therapies, five for solid tumors and seven for hematological malignancies (see Table 1).
Table 1. ADC Approvals
Key considerations for clinical trials involving ADCs
One of the best indicators of the therapeutic potential of a cancer drug is whether it can distinguish a cancer cell from a normal cell. The ultimate goal of an ADC is to use the specificity between antibody and antigen to deliver the payload only into tumor cells that express/overexpress the antigen. Unfortunately, to date, no truly tumor-specific antigen has been identified, and most known antigens are also expressed to varying degrees on normal, healthy tissues. As a result, all currently approved ADCs are associated with some toxicity and three have boxed warnings.
Although the safety profiles of ADCs can be predicted to some degree based on their composition, careful dose selection, close monitoring, and comprehensive adverse event reporting and attribution are essential in first-in-human clinical trials. In addition, there may be a need to observe patients after administering ADCs to monitor for and manage any infusion-related side effects for the first time. This need for extended observation must be factored into site feasibility, as some sites may not have the space or resources to accommodate this requirement.
Patient selection in clinical trials may also be a challenge. In many solid tumors, target antigen expression is highly heterogeneous and may change over time.2 It may be necessary to not only measure target antigen expression in tumor tissue, but also determine cut-offs for antigen positivity.
Future trends
The full potential of ADCs is yet to be realized. In addition to further innovations in the design of these drugs to optimize efficacy and safety, the years to come will bring investigations into therapeutics that can be combined with ADCs to augment clinical efficacy. One emerging strategy involves using partner drugs to modulate the expression of the target antigen, though off-target expression in non-malignant cells may also potentiate toxicity.2 ADCs are also being studied in combination with immunotherapies based on the rationale that ADC-mediated cell death might prompt immune effector cells to recognize immunologically cold tumors. Beyond oncology, ADCs may also have therapeutic potential in antibiotic-resistant infections and autoimmune disease.
Conclusion
ADCs are complex therapeutics with unique pharmacokinetic profiles and mechanisms of action and resistance that are yet to be fully understood. These drugs represent an exciting, but still relatively young, area of research with the potential to advance the field of targeted therapy for cancer. At Premier Research, we understand the opportunities and complexities associated with the development of ADCs in oncology and have experience with both early- and late-phase clinical trials of those novel therapeutics.
[1] Theicher BA, Chari RV. Antibody conjugate therapeutics: challenges and potential. Clin Cancer Res. 2011;17:6389–6397.
[2] Drago JZ, Modi S, Chardarlapaty S. Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat Rev Clin Oncol. 2021;18(6):327-344.
[3] Alley SC, Okeley NM, Senter PD. Antibody-drug conjugates: targeted drug delivery for cancer. Curr Opin Chem Biol. 2010;14:529-537.
