Welcome to part two of our blog series focused on the integration of diversity, equity, and inclusion (DEI) into clinical research. If you missed part one of the series that highlights recent FDA initiatives and legislative changes behind these developments, click here.
In today’s clinical research environment, sponsors face the challenge of comprehensively incorporating DEI into their clinical trials, including design, recruitment strategies, and overall management. Developing a robust DEI strategy involves understanding disease prevalence among different demographic groups, setting enrollment goals, and addressing engagement and recruitment strategies. In this blog, we review why DEI is so important in clinical research and outline four key steps to developing a DEI plan that addresses FDA requirements. Stay tuned for part three of this blog series, which will provide an overview of regional differences to consider when preparing to implement a DEI plan.
The Importance of Diversity
- The value of representation: Including diverse participants in clinical trials ensures that the findings are reflective of the broader population, particularly when diseases and conditions affect different demographic groups in distinct ways.
- Propelling precision medicine: A diverse participant pool allows researchers to identify patterns and responses specific to different groups, paving the way for more effective and personalized healthcare.
- Ethical considerations: Excluding certain populations from research can perpetuate healthcare disparities and exacerbate existing inequalities in access to innovative treatments.
The Role of Equity
- Research access: Addressing barriers such as language and socio-economic factors is important for ensuring equitable access to clinical research opportunities and building trust within marginalized communities.
- The benefit of patient-centric design: Considering the diverse needs of participants, including factors like transportation, childcare, and flexibility in scheduling, will help barriers to participation.
The Power of Inclusion
- Community engagement: Engaging with communities throughout the research process fosters trust and collaboration. Involving potential participants in study design, recruitment strategies, and sharing results ensures that research aligns with needs and concerns of patients in the real world.
- Addressing bias: Inclusive research practices can help identify and mitigate biases in study design, participant selection, and interpretation of results, driving more robust and reliable research outcomes.
4 Steps to Incorporating DEI into Clinical Trial Design and Recruitment
To create a solid DEI plan that addresses FDA requirements, sponsors must consider:
- Disease overview and prevalence analysis: It is essential to understand how the disease impacts different demographic groups. This requires an exhaustive analysis of the disease condition, focusing on its prevalence among underrepresented communities.
- Study design and population eligibility: Clinical development experts must explore study designs, population eligibility criteria, and endpoints through a DEI lens. This includes examining geographic locations and ensuring they contribute to the diversity of the study population.
- Setting enrollment goals: Establishing clear, achievable targets for the enrollment of underrepresented racial and ethnic groups is crucial. This involves developing specific plans and strategies to meet these goals including the collection of pharmacokinetic, pharmacodynamic, and pharmacogenomic data that may impact how trial participants respond to drugs.
- Addressing engagement and recruitment strategies: The FDA requires a comprehensive approach to recruitment and engagement strategies. This includes addressing factors such as skin pigmentation that may impact device performance and considering how race and ethnicity could affect drug or device efficacy and safety. Collecting and analyzing data on potential efficacy differences related to race or ethnicity is encouraged throughout the product lifecycle.
The Role of Cross-Functional Collaboration in DEI
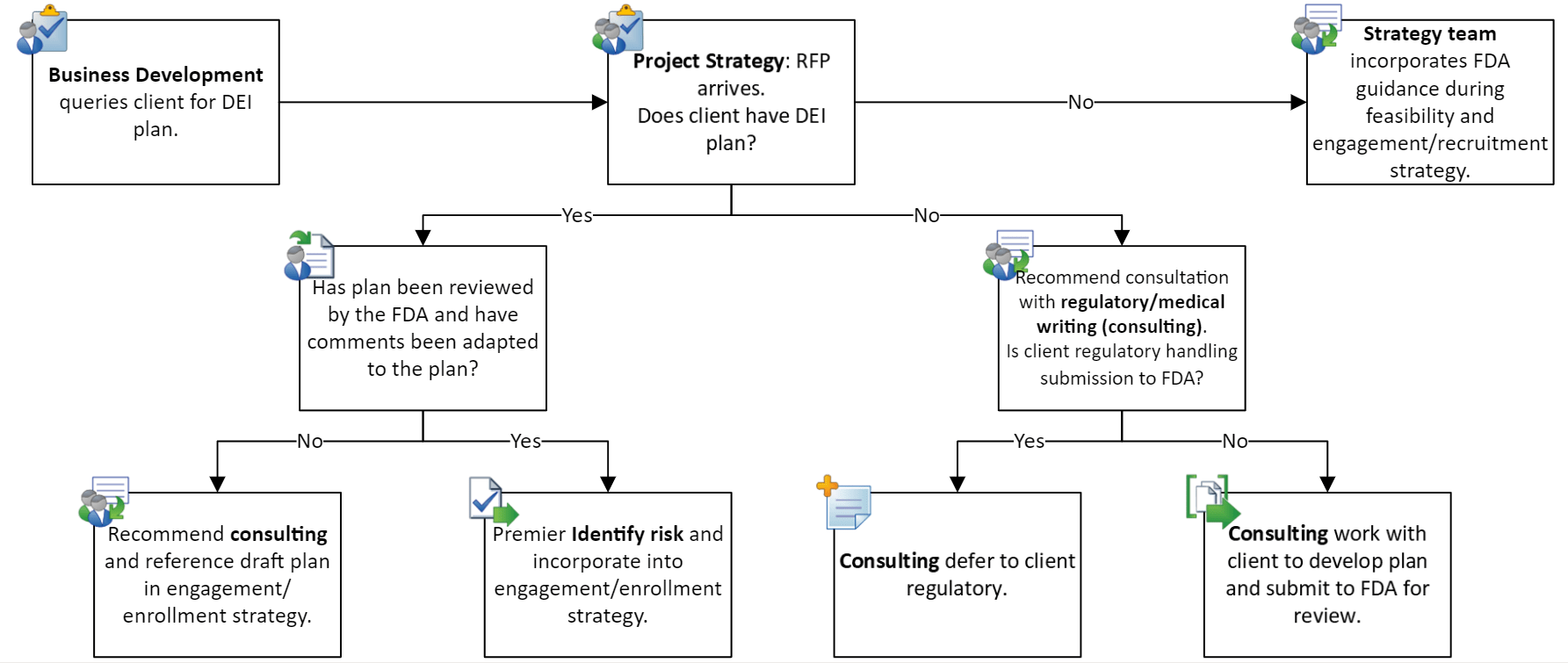
Integrating DEI into clinical trials is not a solitary endeavor but a cross-departmental effort. It necessitates collaboration among various departments, including medical writing, risk management, consulting, and operations.
Premier Research’s goal is to help sponsors develop a unified strategy that not only adheres to regulatory standards but genuinely embodies a commitment to DEI.
Figure 1: Premier Research Process for Ensuring Sponsors Address DEI Requirements
DEI: A Pillar for Progress
Embracing DEI in clinical research is now not only a moral imperative but a regulatory requirement as sponsors develop their overarching clinical development plans. This shift towards inclusivity will not only advance the scientific understanding of diseases and treatments but also contribute to a more equitable and just healthcare system. To learn how Premier Research can support you with your DEI plans, contact us.