Dermatology
With Ever-Increasing Treatment Innovations, Dermatology Conditions Demand Knowledge that Goes Beyond the Surface
Our Expertise in Dermatology is Clear
Faster Trial Start-up and Better Patient Outcomes
Advances in treating skin diseases are changing lives, and we’re leading this fast-moving field with expertise across most major dermatological indications. Having performed more than 200 dermatology studies and worked with thousands of patients over the past five years, we know how to make your study a success.
From mild to moderate acne and eczema to life-threatening cases of metastatic melanoma, new treatments are increasing the accuracy and efficacy of treatments while reducing side effects. Our clinical trial expertise helps sponsors realize the potential of these promising innovations.
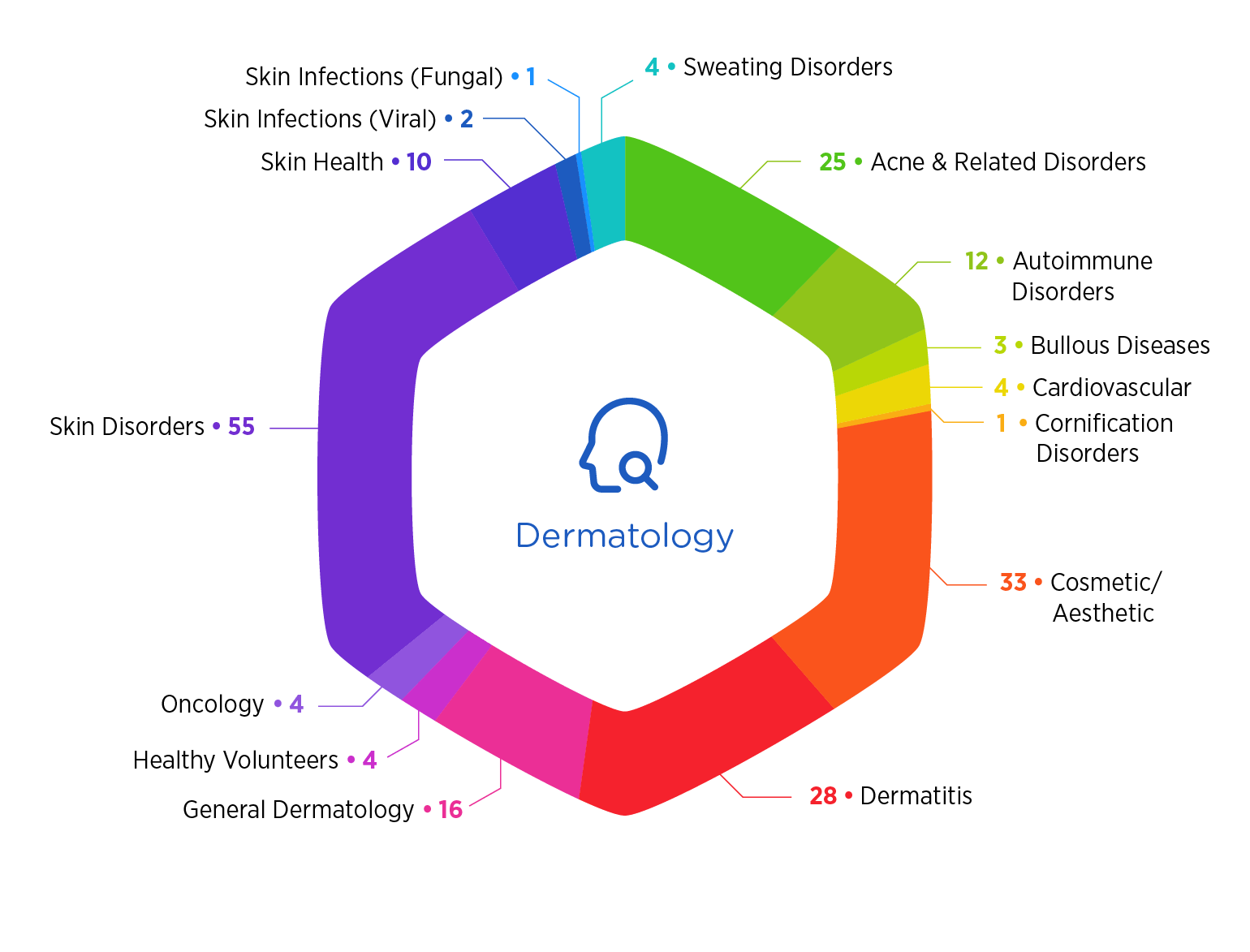
Why Choose Premier
- We’ve studied more than 13,200 patients at hundreds of sites
- Our experience enables us to accelerate timelines by up to 25 percent through rapid start-up and recruitment
- Specialties include pediatrics, from neonates to adolescents
Agility and knowledge at your fingertips
Resources

Guides
Expert Guide to Decentralized Dermatology Trials: Five Strategies for Study Success
To optimize the likelihood of success, dermatology clinical trials should be designed with the patient in mind, minimizing the burden of participation and maximizing accessibility for a diverse population of qualified patients.

Perspectives Blogs
5 Key Takeaways: Drug and Device Development Secrets in the World of Medical Aesthetics
New treatment options for medical aesthetic indications are in record-high demand, fueled by growing awareness of the effects of physiological and environmental aging and the influence of lifestyle on skin health.
Meet the Experts
Dermatology