Rare Disease
Tackling Unmet Needs Handcuffed by Small Populations, Limited Data, and Evolving Regulations is Nothing Rare for Us
Accelerating Rare Disease Research
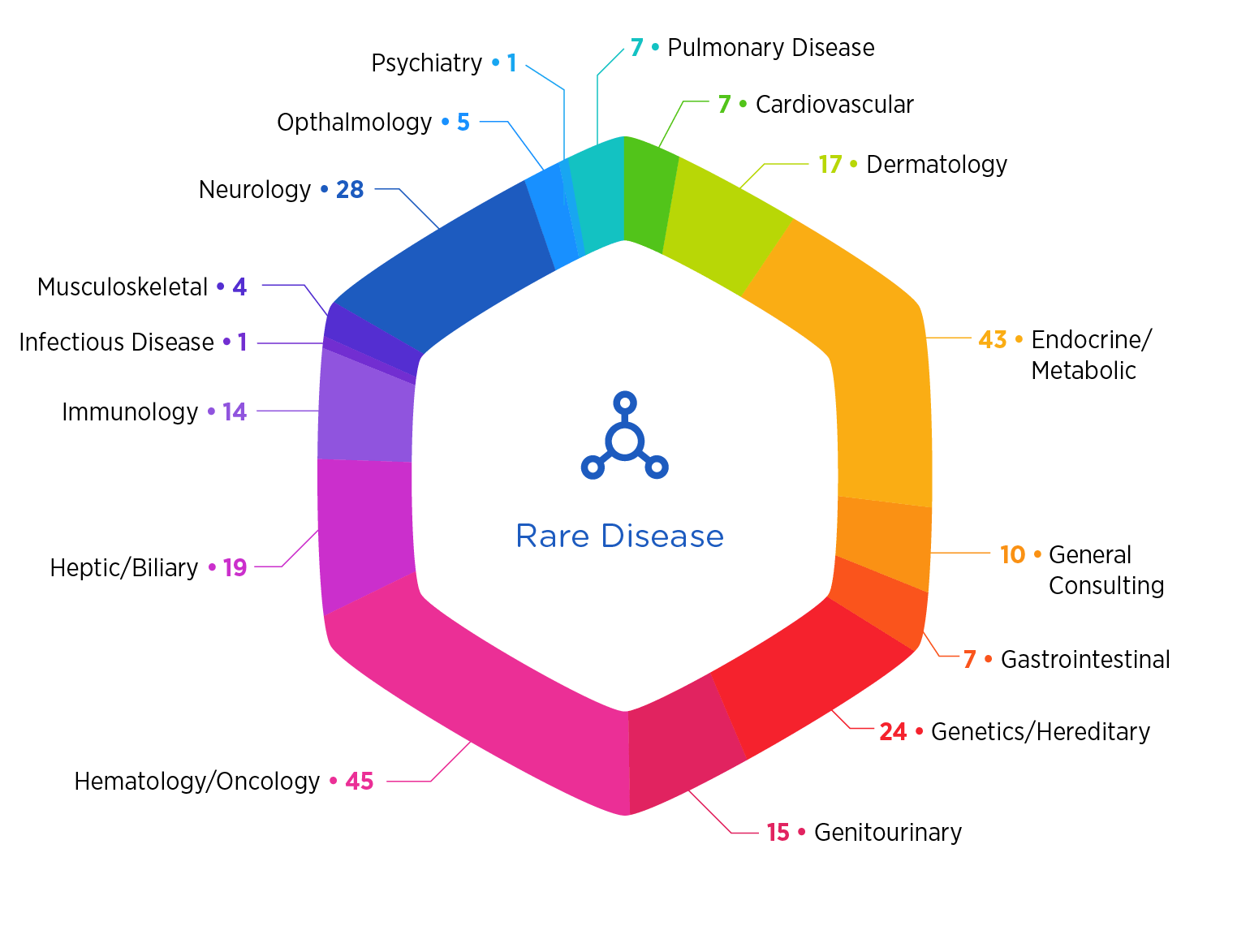
With decades of hands-on experience in rare diseases across 88 indications — including rare cancers and ultra-rare conditions — we propel you from innovative concept to commercial reality with product development, regulatory, nonclinical, and clinical trial solutions.
Thriving Within Complexity
A brilliant idea is just the beginning of a complex journey from compound to groundbreaking treatment. We are built for this complexity, delivering a continuum of fit-for-purpose rare disease solutions — from regulatory and product development consulting to smart study design and proven strategies for accelerating start-up and enrollment, maximizing patient engagement, and conveying clinical trial transparency with real-time data.
Innovating Through Strategic Collaborations and Advanced Technologies
Embracing the unknown is easier when we take it on together. That’s why we partner with biodatabanks, multiomic reference laboratories, and leaders in advanced computational modeling and simulation, collaborating to fast-track and de-risk rare disease clinical development. These exceptional partnerships and our deep connections with advocacy groups, KOLs, investigators, and sites enhance how we design and recruit for rare disease clinical trials.
Unparalleled Experience in Navigating Uncharted Waters
We provide end-to-end solutions for single- or multi-asset innovators across all phases of product development:
- Focused regulatory guidance. Navigate the shifting landscape and optimize regulatory pathways through our expert consultation and well-established relationships with regulators to achieve orphan drug designation and accelerate approval.
- Innovative study design and endpoint selection. Small patient populations pose large challenges. Our significant experience with natural history studies, in silico modeling, and real-world data planning makes the most of limited data while satisfying regulatory requirements.
- Partnership with trial participants. Relationships with clinical trial participants and their caregivers must begin before — and extend beyond — enrollment. Our proven strategies throughout the development process generate deeper understanding of the disease and its challenges, more meaningful endpoints, and stronger study engagement.
Agility and knowledge at your fingertips
Resources
Rare Disease Research Embraces the Unknown. Let’s Take It On. Together.

8 Cost Containment Strategies for Rare Disease Clinical Trials
Meet the Experts
Rare Disease

Angi Robinson