Cell and Gene Therapies
From Stunningly Complex Science to Life-Changing Treatments
Extending Hope to Those Most in Need
Cell and gene therapies are at the forefront of scientific advancement for those patients most in need of hope. That science and those patients are at the very heart of our passion for cell and gene therapy development. With obstacles ranging from regulatory and nonclinical strategy, to CMC considerations and immunogenicity, to start-up regulations, patient recruitment, logistics, and long-term follow-up, our multidisciplinary team delivers for biotech innovators from start to finish.
Mitigating the Intricacies of Cell and Gene Therapies
Cell and gene therapies diverge from small molecules not only in their design, but also in the skills, capabilities, and relationships needed to transition these advanced therapeutics from concept to commercialization. We bring both depth and breadth of expertise in cell and gene therapy, integrating the full development life cycle across therapeutic indications and treatment modalities. From regulatory strategy, nonclinical services, and medical expertise to meticulous logistics management, seamless clinical delivery, and proactive safety monitoring, we enable the science that’s behind the vast promise of cell and gene therapy.
Unrivaled Experience Paving the Way to Success
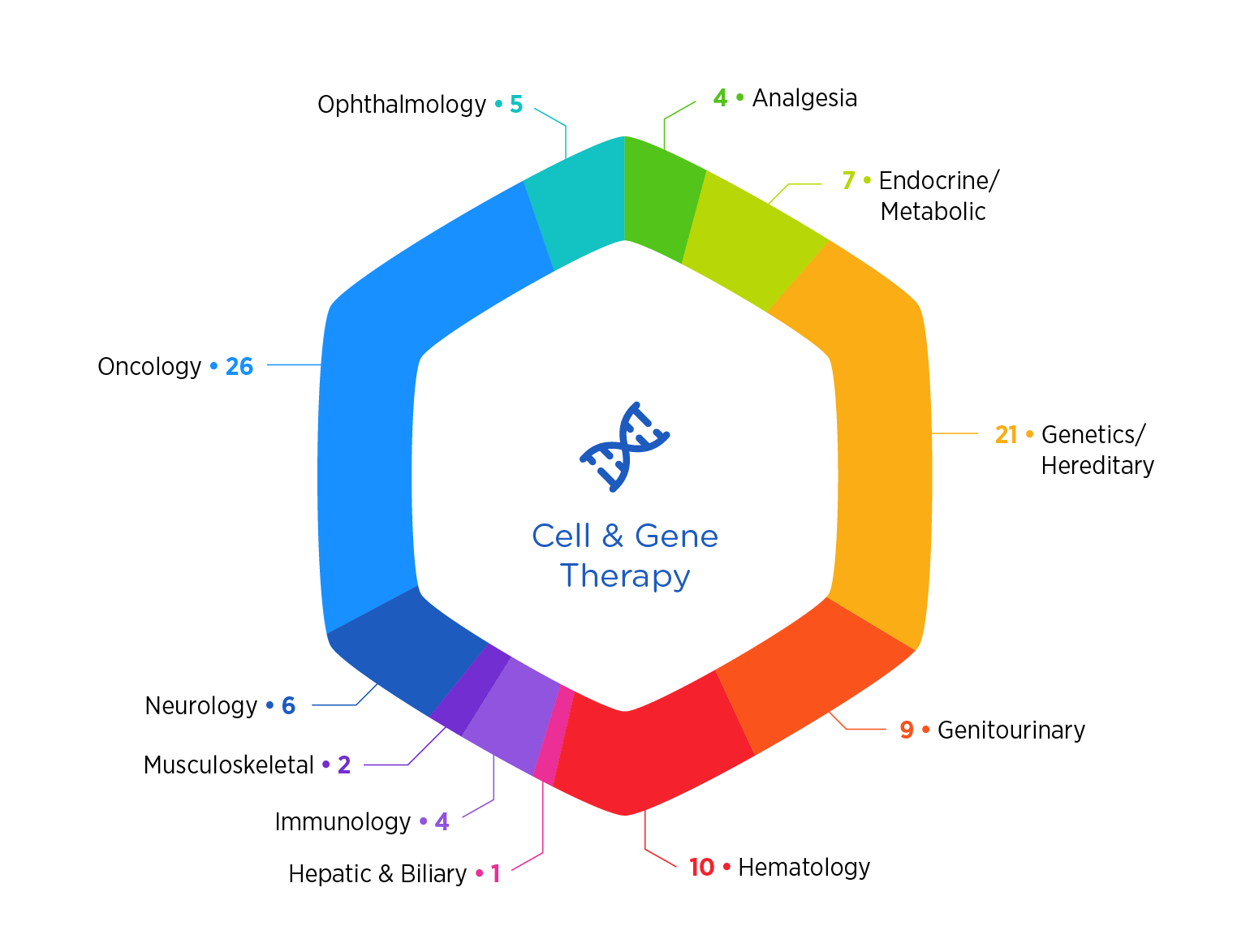
From safety considerations to complex vein-to-vein logistics, the breadth of our experience across molecule types and therapeutic areas differentiates us. With nearly 100 CGT projects conducted globally in the last five years, we offer significant experience across a broad range of CGT modalities inclusive of in vivo gene therapies (e.g., AAV) and ex vivo gene modified cell therapies (e.g., CAR-T, HSCs).
Our broad experience across both viral and non-viral vectors (e.g., AAV, LVV, Adenovirus, LNPs), therapeutic areas (e.g., Rare Disease, Oncology) and administration routes (e.g., IV, intrathecal, intracerebroventricular, intracisternal) provides you with a partner that has seen it all and done it all. From early preclinical to late-stage development, we know that a holistic approach to CGT development is critical to success. We assemble highly specialized, cross-functional teams to support the innovators developing these novel therapies.
- Robust Safety Monitoring
Extensive experience assessing and monitoring patient safety to address potential concerns around prolonged biologic activity, immunogenicity, and invasive procedures. - Fully-Integrated Logistics Management
Strategies to reduce workflow risk in vein-to-vein logistics across both allogeneic and autologous cell therapies. - High-Touch Patient Experience
Patient-centric delivery ensures safety, minimizes inconvenience, encourages completion, and simplifies long-term follow-up to reduce patient burden and improve retention. - Rigorous Scientific Consulting
Proven regulatory and submission guidance keeps pace with dynamic global standards to move breakthrough therapies forward, while innovative study designs, such as those that incorporate real-world data and in silico modeling, help to accelerate product approval. Expert CMC considerations to manage the complexities of developing CGT products.
Agility and knowledge at your fingertips
Resources

In Silico Trial Design in Development of Rare Disease Cell and Gene Therapies

Long-Term Follow-Up in Gene Therapy Trials: Ensuring Patient Engagement & Regulatory Compliance
Meet the Experts
Cell & Gene Therapy