Neuroscience Clinical Trial Experience
Unraveling the Field’s Complexity with an Understanding that Comes From Experience
Breaking Barriers in Neuroscience
A Nuanced Approach to a Uniquely Complex Field
These are exciting times for neuroscience drug development as we help the most innovative sponsors unlock the mysteries of the central nervous system (CNS) and bring breakthrough therapies to millions of patients in need. From our pioneering work in rare diseases to the growing field of consciousness-expanding psychedelic drugs – where our experience in licensing, regulation, and investigator and therapist training is helping shape a new class of therapies – our neuroscience clinical research and regulatory professionals are committed to supporting you at any point of your development journey.
Having performed more than 350 projects across CNS research in the past five years, our team of experts offer a diverse range of psychiatric and neurological experience to provide the best possible regulatory and scientific, medical, and operational delivery for the success of your asset. As your novel therapy moves from concept to commercialization, we have the hands-on experience and knowledgeable staff to provide the precise approach you need to anticipate and overcome obstacles along the way.
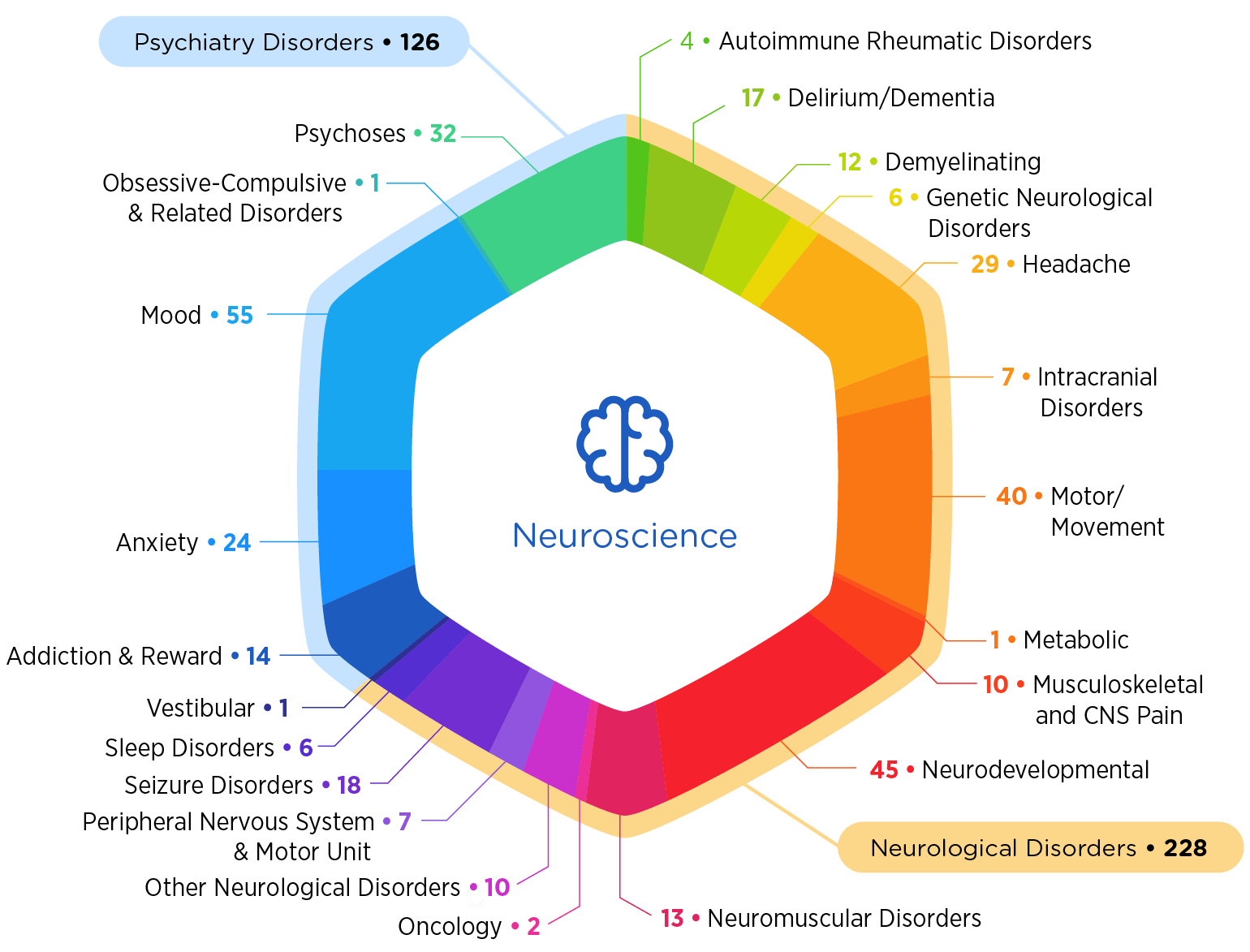
Why Choose Premier
- Our work has touched more than 50,000 patients afflicted by just about every major central nervous system disorder
- More than half of our work is in rare diseases
- We have a history of breakthrough work, including the first post-traumatic stress disorder study in military veterans
Premier Rater Training, Scale Management, and Clinician Led Endpoint Surveillance
- Available across Premier neuroscience studies in multiple indications
- Strategic focus to build upon our previous success of conclusive studies
- Utilize feedback from sponsors and sites to deliver quality, timely rater training & site activation
- Unique capabilities for endpoint quality management (both clinical and logistic considerations to maximize success)
- Incorporated into overall study quality strategy including clinical and medical monitoring
Agility and knowledge at your fingertips
Resources

Premier Insight 278: A Decade-Long Partnership Culminates in FDA Approval

Premier Insight 273: Conclusive & Positive Results Delivered Ahead of Time – By Eight Months
Meet the Experts
Neuroscience