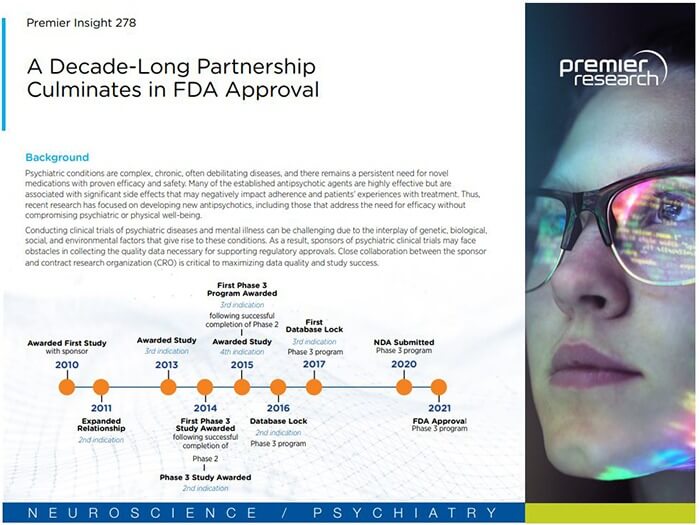
Background
Psychiatric conditions are complex, chronic, often debilitating diseases, and there remains a persistent need for novel medications with proven efficacy and safety. Many of the established antipsychotic agents are highly effective but are associated with significant side effects that may negatively impact adherence and patients’ experiences with treatment. Thus, recent research has focused on developing new antipsychotics, including those that address the need for efficacy without compromising psychiatric or physical well-being.
Conducting clinical trials of psychiatric diseases and mental illness can be challenging due to the interplay of genetic, biological, social, and environmental factors that give rise to these conditions. As a result, sponsors of psychiatric clinical trials may face obstacles in collecting the quality data necessary for supporting regulatory approvals. Close collaboration between the sponsor and contract research organization (CRO) is critical to maximizing data quality and study success.