Last Updated: May 28, 2025, 2 pm UTC
In an industry that has historically relied on manual oversight methods, clinical trial monitoring is evolving—but not rapidly. Over the past several decades, the implementation of technology and risk-based monitoring (RBM) strategies has become essential for ensuring both data integrity and patient safety—however inefficiencies of traditional 100% source data review (SDR) and source data verification (SDV) continue to be the primary method for monitoring. Despite clear regulatory endorsement and scalable technologies, many in the industry remain tied to outdated practices including 100% SDR/SDV (see Figure 1).
Figure 1: RBQM landscape over 5 years: ACRO
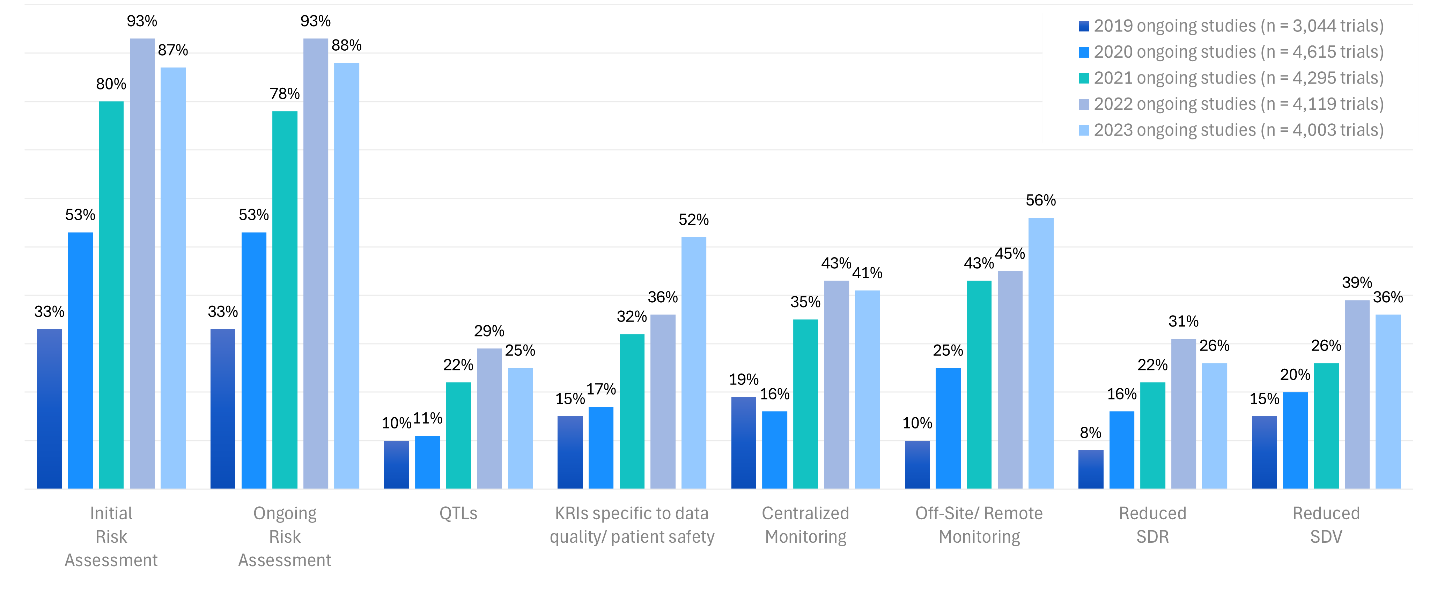
Source: https://www.acrohealth.org/rbqm-summary-report/
Since 2007, when discussions around clinical trial efficiency gained traction through initiatives like Clinical Trial Transformation Initiative (CTTI), there has been a growing recognition that increased technology should equate to increased operational efficiency. Organizations such as ACRO (Association of Clinical Research Organizations), in collaboration with TransCelerate and regulatory stakeholders, have worked to promote adoption of integrated quality risk management (IQRM) approaches such as centralized monitoring and SDR/SDV sampling strategies.
This blog examines how centralized monitoring and SDR/SDV sampling strategies are transforming trial oversight through data integration, targeted review strategies, and advanced analytics. These modern approaches not only enhance patient safety and trial quality but also drive cost efficiencies by enabling early risk detection, reducing burden of on-site monitoring, and optimizing resource allocation.
IQRM: A three-pronged approach
IQRM can be broken down into three essential components:
- Risk assessment and mitigation planning – Widely adopted across the industry, this process identifies critical data and processes, evaluates what can go wrong, and outlines strategies to accept, mitigate, or monitor those risks.
- Centralized monitoring – This strategy includes aggregating data captured across multiple data sources such as EDC, labs, and ePRO to enable real-time visibility across patient-level and study-level data, allowing for proactive issue identification. Adoption of centralized monitoring has steadily increased, especially since the onset of the COVID-19 pandemic.
- Risk-based monitoring – This strategy includes sampling data at a data point, procedure/form, patient visit or patient level. Despite growing evidence and regulatory encouragement, the industry continues to struggle with moving away from traditional 100% SDR and SDV. This reluctance represents a significant opportunity for improvement.
Centralized monitoring in action
Central monitoring allows stakeholders to review aggregated data from multiple sources (EDC, labs, ePRO, wearables, etc.) in near real-time (see Figure 2). Dashboards provide both patient-level and study-level visibility, offering early detection of trends, deviations, non-compliance, and data anomalies—often within five days of data entry.
Figure 2: Central Monitoring Process
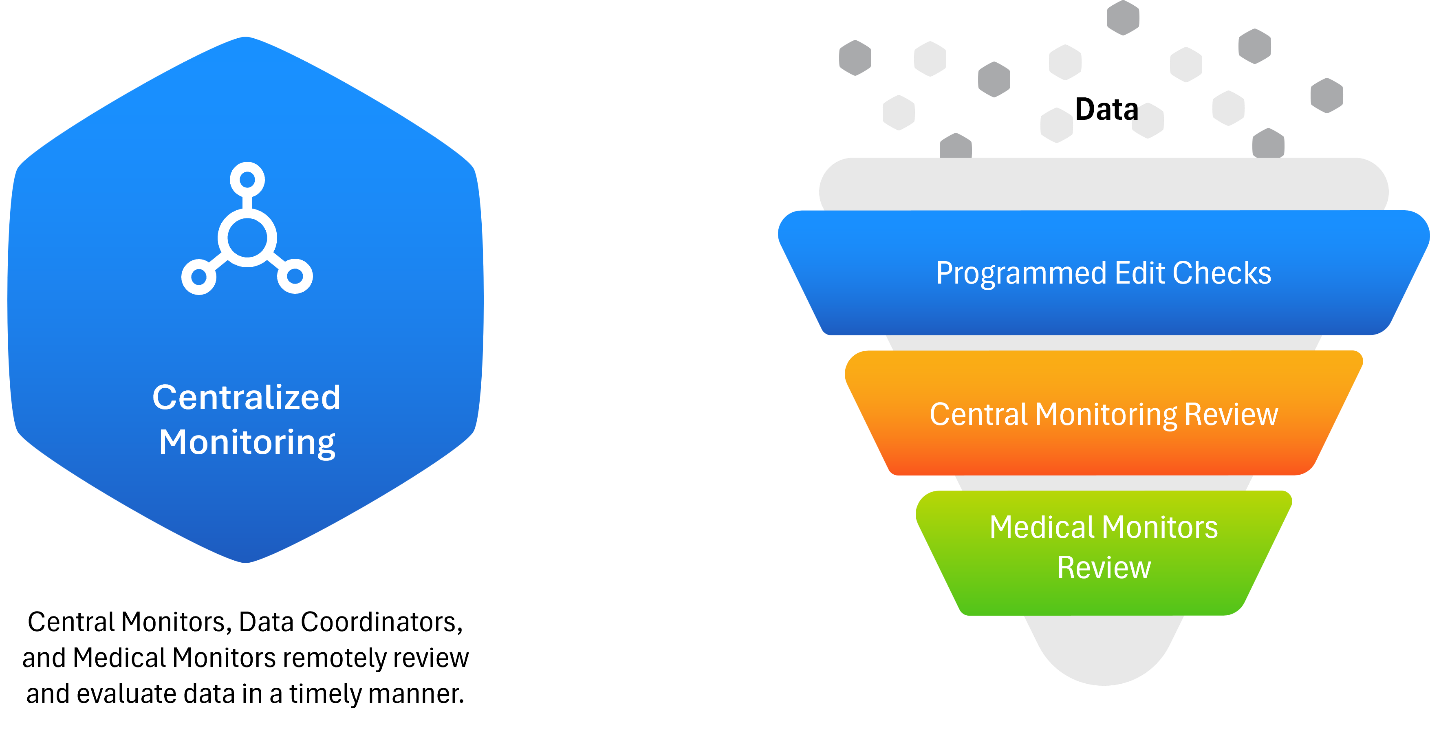
This approach facilitates rapid issue identification, allowing clinical teams, data management, and medical monitors to collaborate efficiently. Instead of combing through all patient data manually, centralized teams can triage and focus on data that signals risk, improving both speed and accuracy.
Key advantages of centralized monitoring
- Speed: Central monitors detect approximately 40% of protocol deviations—20% faster than CRAs conducting on-site visits.
- Scalability: Effective even in small trials; no longer restricted to large, multi-site studies.
- Efficiency: Reduces dependence on manual review, streamlines communication, and provides traceability for findings and resolutions.
Medical reviewers also benefit by focusing on flagged patients rather than all subjects, enhancing their ability to provide targeted insights and maintain patient safety.
The corn analogy: Rethinking traditional monitoring
Traditional clinical trial monitoring is akin to walking through a vast cornfield and inspecting every kernel on every cob — a time-intensive and resource-heavy approach that is neither scalable nor efficient. In this analogy, each corn stalk represents a patient, each corn cob represents a patient visit, each row of kernels on a cob represents a procedure, and each kernel represents an individual data point (see Figure 3). Attempting to examine every kernel reflects the outdated model of 100% SDV and SDR, which, while thorough, is not sustainable for today’s complex trials.
Figure 3: Traditional monitoring cornfield analogy
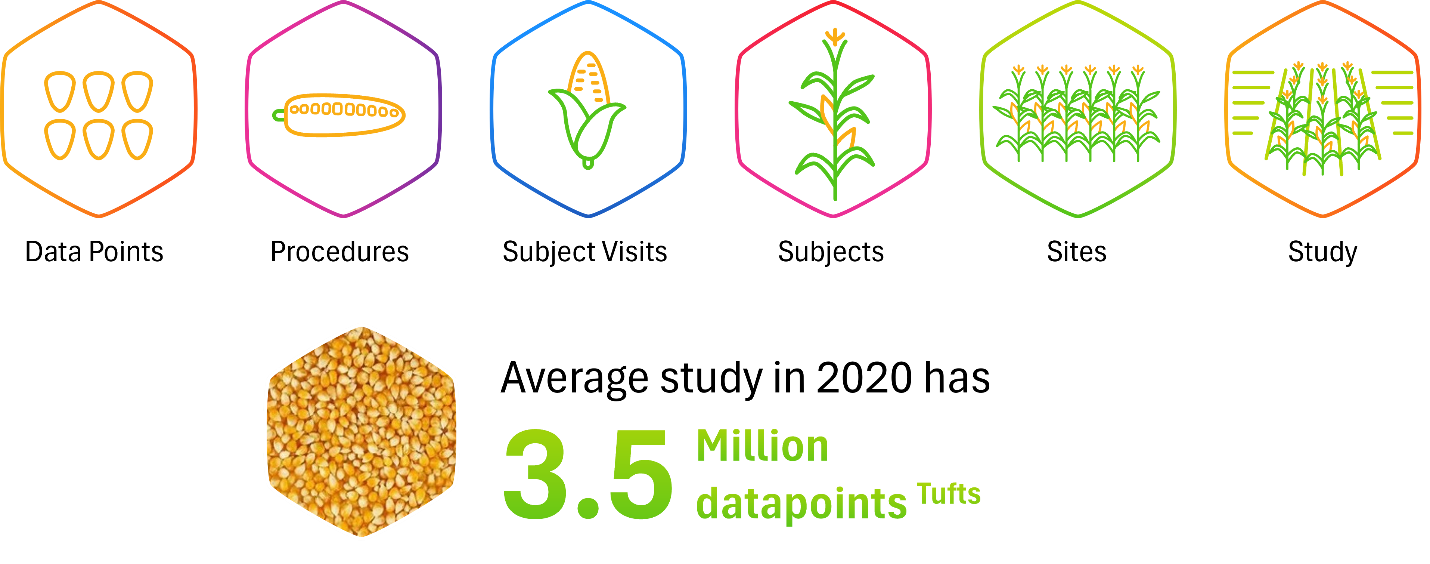
Centralized monitoring transforms this paradigm. Rather than inspecting every kernel manually, it’s like deploying a high-resolution drone over the field. From this aerial vantage point, patterns and anomalies can be quickly identified — clusters of unhealthy crops, areas of underperformance, or outliers that warrant closer inspection. In a clinical context, this translates to using aggregated data, analytics, and automated triggers to detect signals of risk or non-compliance early. The “drone” (i.e., centralized monitoring platform) guides clinical research associates (CRAs) to focus their SDR/SDV efforts where the data suggest there may be problems, thereby directing resources efficiently.
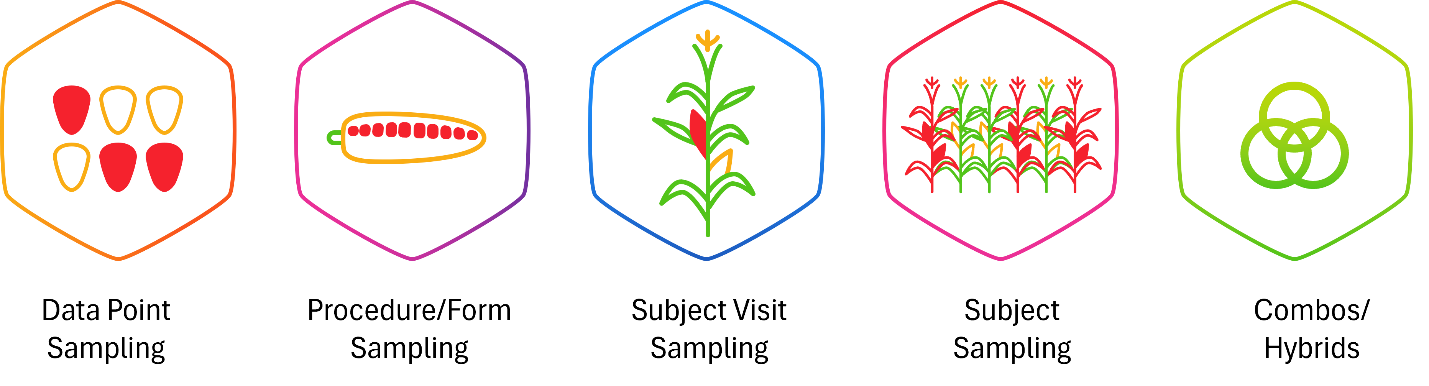
Building on the analogy, sampling strategies function like targeted spot checks within the field. Rather than inspecting every kernel on every cob on every plant, monitors select representative patient visits (or cobs) to assess quality (see Figure 4). These include:
- Data point sampling: Reviewing only specific kernels based on predefined criteria or risk indicators.
- Procedure/form-level sampling: Focusing on certain groups or rows of kernels on each cob.
- Subject visit sampling: Checking entire cobs for every patient (all data from a sampling of visits).
- Subject sampling: Choosing a subset of plants across the field.
- Hybrid models: Combining multiple sampling strategies to optimize coverage based on the terrain and observed risks.
Figure 4: Different sampling approaches
These targeted methods reduce unnecessary review burden and cost while maintaining a high standard of quality oversight — much like a farmer who uses precision agriculture to manage crops efficiently and cost-effectively. By moving away from exhaustive review and toward intelligent, risk-informed monitoring, sponsors can optimize operations, conserve resources, and improve trial outcomes.
Regulatory endorsement and the path forward
Since 2006, regulators have encouraged the industry to adopt a more risk-based, sampling-focused approach. Despite this, the vast majority of RFPs still request full SDR/SDV—highlighting a persistent gap between technological capabilities and legacy practices.
To bridge this gap, sponsors and CROs must champion the shift toward IQRM, investing in stakeholder education and implementing scalable, data-driven tools that support centralized monitoring and intelligent sampling. This evolution isn’t about doing less—it’s about working smarter.
Just as modern agriculture relies on drones and data to manage fields more efficiently, clinical research must embrace a precision approach to oversight. Centralized monitoring and sampling models not only align with regulatory expectations but also deliver measurable cost savings, reduce operational burden, and improve data quality. In a resource-constrained environment, adopting targeted, risk-based strategies is no longer optional—it is essential to ensuring both scientific integrity and trial sustainability.
To learn about how we deploy and manage centralized monitoring or SDR/SDV sampling strategies, contact us.
ABOUT PREMIER RESEARCH:
Premier Research, a global clinical research, product development, and consulting company, is dedicated to helping innovators transform life-changing ideas and breakthrough science into new medical treatments. We offer strategic solutions across the entire development lifecycle, from pre-clinical through commercialization, specializing in smart study design and full-service clinical trial management.
Leveraging technology and therapeutic expertise, we deliver clean, conclusive data with a focus on reducing development timelines, securing access to the right patients, and effectively navigating global regulations to ensure submission-ready results.
As an organization that puts patients first, we pride ourselves on helping customers answer the unmet needs of patients across a broad range of medical conditions. Visit premier-research.com.

 Webinar
Webinar 


 Perspectives Blog
Perspectives Blog 