Last Updated: August 6, 2025, 8 am UTC
With over 1 billion individuals affected globally, obesity has emerged as one of the most pressing public health challenges of our time (see Figure 1). Increasingly, the global health community is recognizing obesity not merely as a lifestyle condition but as a complex, chronic disease requiring long-term, multidisciplinary management. However, despite this growing consensus, the path toward effective and equitable treatment remains fraught with clinical, structural, and societal challenges.
Figure 1: Obesity in Adults, 1975 to 2016
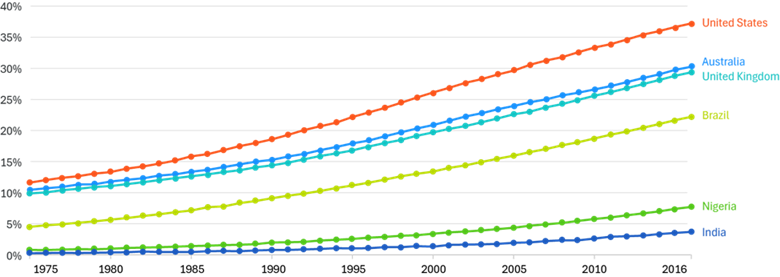
Source: World Health Organization – Global Health Observatory (2024)
Therapeutic innovation in obesity treatment is advancing rapidly, led by the presence in the market of GLP-1 receptor agonists and dual agents such as semaglutide and tirzepatide. These agents are reshaping the treatment landscape, demonstrating not only significant weight loss outcomes but also improvements in broader cardiometabolic health. Industry analysts estimate that the global market for obesity therapeutics could exceed $130 billion by 2030i. Yet this rapid expansion prompts a critical consideration: can existing care delivery systems and clinical research infrastructure adapt swiftly enough to support and sustain these advancements?
The Race for Oral Administration and Multi-Receptors Agonism
While GLP-1 receptor agonists are expected to remain a cornerstone of obesity pharmacotherapy, the development pipeline is expanding to include novel mechanisms that may broaden treatment options and enable more tailored care (see Figure 2).
Figure 2: Obesity Pipeline
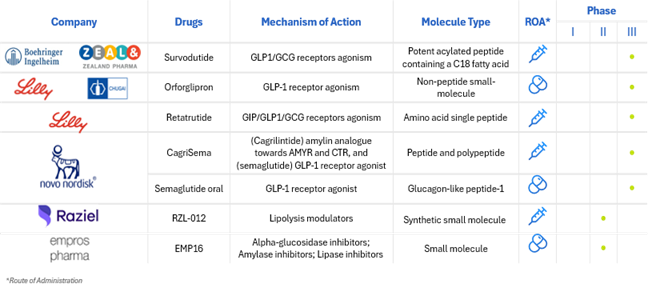
Source: Adapted from https://www.delveinsight.com/blog/emerging-therapies-for-obesity-treatment
Among the most promising are agents targeting entero-pancreatic hormones such as GIP, amylin, and peptide YY. Tirzepatide, a dual GIP and GLP-1 receptor agonist, has already demonstrated substantial clinical success, and GIP monotherapy is under early investigation for its potential to induce weight loss with fewer gastrointestinal side effects. Amylin analogs, which enhance satiety and glucose regulation, are being refined beyond earlier iterations like pramlintide for improved tolerability in obesity treatment. Likewise, peptide YY (PYY) agonists, which reduce appetite via central Y2R receptor pathways, are being studied for their synergistic potential when combined with GLP-1 therapies. In parallel, monoclonal antibodies such as bimagrumab are being explored for their ability to preserve lean mass during weight loss—particularly relevant in conditions like sarcopenic obesity.
Finally, early-stage trials are assessing the utility of GDF-15 receptor agonists, which may suppress appetite via central mechanisms and offer new directions for treatment. The emergence of pharmacologic diversity in the obesity pipeline stands to empower clinicians to select therapies based not only on efficacy, but on individual patient profiles, safety considerations, and comorbidity burden—bringing the field closer to truly personalized obesity care.
Scientific Breakthroughs, Systemic Barriers
Despite promising advancements, access to obesity treatments is still hindered by cost, coverage limitations, and persistent stigma. In a recent survey, 52% of respondents identified insurance coverage as the top barrier to careii. Many private plans exclude obesity medications outright, and political opposition has stymied efforts to expand Medicare coverage.
These coverage gaps leave patients facing out-of-pocket costs that can exceed $1,000 a month—unsustainable for most, especially considering obesity disproportionately affects individuals from lower socioeconomic backgrounds. For many, these barriers are not abstract. They translate into daily decisions—choosing between medication and other essentials or cycling on and off treatment depending on affordability. This financial volatility not only jeopardizes individual health outcomes but also skews the broader picture of treatment efficacy, as adherence and continuity become inconsistent across populations.
Compounding the issue, not all healthcare providers are adequately trained in the nuances of obesity care. Without consistent clinical guidance, patients may be misinformed, misdiagnosed, or under-supported in their treatment journeys.
At the same time, societal stigma continues to color perceptions of obesity, influencing everything from media narratives to clinical interactions. Stigma doesn’t just harm patients—it also undermines research. Individuals may hesitate to disclose their condition, let alone enroll in clinical trials, out of fear of judgment or previous negative experiences.
Why Clinical Research Must Evolve
For clinical development to reflect the reality of living with obesity, trial design must evolve—urgently and intentionally. Sponsors and sites face mounting challenges in recruiting and retaining participants for obesity trials. Treatment-naïve patients are increasingly difficult to find, as more people begin using medications off-label or via telehealth services. Retention is also a persistent hurdle, with dropout rates often exceeding 30%, driven by factors like side effects, logistical burdens, and unrealistic expectations.iii
Traditional trial designs often fail to reflect the lived experiences of individuals with obesity. Protocols may be overly restrictive, exclude participants with common comorbidities, or require frequent in-person visits—barriers that disproportionately affect underrepresented groups and limit real-world applicability of findings.
Building trust is key. And that trust doesn’t come from recruitment materials or scripted site visits alone—it comes from designing trials that center the full patient experience.
Centering the Patient Through Integrated, Multidisciplinary Care
Obesity affects nearly every aspect of a person’s health—cardiovascular, hormonal, psychological, and behavioral (see Figure 3). Treating it effectively requires a care model that does the same. That means moving away from siloed interventions and toward integrated, multidisciplinary approaches that mirror how obesity is (or should be) managed in clinical practice.
Think of obesity care as an orchestra. When each specialty—nutrition, cardiology, endocrinology, behavioral health—plays in harmony, the result is a more complete and compassionate treatment experience. Clinical trials should strive for the same coordination.
Figure 3: Complications Associated with Obesity
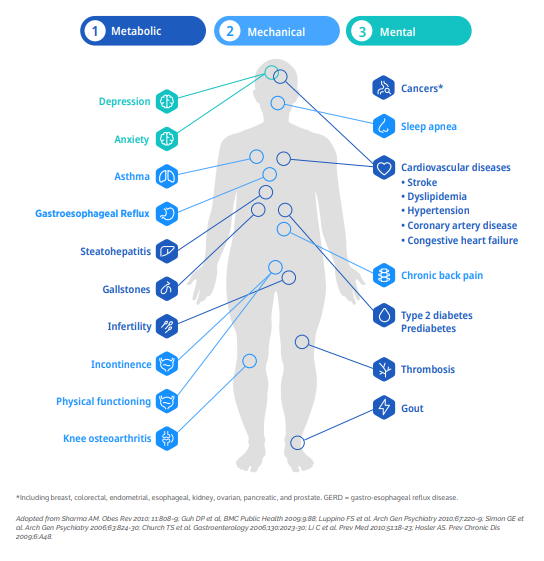
For research teams, this shift offers a critical opportunity: when care teams include endocrinologists, cardiologists, psychologists, and dietitians—not just investigators—patients are more likely to feel supported, engaged, and understood. That translates into higher-quality data, improved retention, and trials that yield insights applicable to real-world settings.
Premier Research is helping sponsors adopt this model through a center of excellence that brings together medical, operational, and behavioral expertise to tailor trial design from the outset.
Looking Ahead: Innovation with Impact
As new oral medications and combination therapies continue to emerge, the obesity treatment landscape is on the brink of a paradigm shift. But unless the research and care models evolve alongside these therapies, the full promise of innovation will remain out of reach.
To move forward, the industry must do more than develop better drugs. It must also create better trials—ones that account for the complexity of obesity and the lived realities of those affected. That means designing with empathy, enabling access, and embedding care into the research experience.
Progress in obesity treatment must be measured not only by scientific advances but by how broadly and fairly those advances are delivered. Equity must be built into every stage of development—from patient selection to outcome measurement—to ensure innovation benefits all populations, not just those with access.
Because when we treat obesity as the lifelong, multifactorial condition it is, we not only improve trial outcomes—we get closer to delivering real health gains to real people.
To learn how we can optimize your next obesity trial, contact us.
ABOUT PREMIER RESEARCH:
Premier Research, a global clinical research, product development, and consulting company, is dedicated to helping innovators transform life-changing ideas and breakthrough science into new medical treatments. We offer strategic solutions across the entire development lifecycle, from pre-clinical through commercialization, specializing in smart study design and full-service clinical trial management.
Leveraging technology and therapeutic expertise, we deliver clean, conclusive data with a focus on reducing development timelines, securing access to the right patients, and effectively navigating global regulations to ensure submission-ready results.
As an organization that puts patients first, we pride ourselves on helping customers answer the unmet needs of patients across a broad range of medical conditions. Visit premier-research.com.
REFERENCES:
[i] Bloomberg Professional Insights. The $130 Billion Obesity Drug Market Has an Insurance Problem. July 29, 2024. https://www.bloomberg.com/professional/insights/markets/the-130-billion-obesity-drug-market-has-an-insurance-problem/
[ii] Alex Montero, Grace Sparks, Marley Presiado & Liz Hamel. KFF Health Tracking Poll May 2024: The Public’s Use and Views of GLP‑1 Drugs. Kaiser Family Foundation, May 10, 2024.
[iii] Pfizer Stops GLP-1 Obesity Trial Because of High Discontinuation Rates – Pfizer Stops GLP-1 Obesity Trial Because of High Discontinuation Rates

 Webinar
Webinar 


 Perspectives Blog
Perspectives Blog 