Last Updated: October 22, 2024, 1 pm UTC
The increasing complexity of clinical trials requires a re-assessment of the functions of site management and monitoring. Both roles have traditionally been the purview of clinical research associates (CRAs), who have typically spent 60%-80% of their time on monitoring and only 20%-40% on site management.
Traditionally, CRAs have a range of monitoring and site management responsibilities that may include investigational product accountability, site staff training, regulatory document review, source data review (SDR), and source data verification (SDV). Today, this has expanded to include access management and technical support of clinical technologies such as ePROs, eDiaries, imaging systems, wearables, recruitment portals, and many other new and innovative tools and processes.
Moreover, an increased focus on risk-based and central monitoring is decreasing the time CRAs spend onsite, emphasizing the need for robust site management. Additionally, experienced human resources are scarce and costly to deploy in global trials where CRAs are needed at the right time and in the right location, depending on each site’s pace of enrollment and volume of patients and data. This can be especially complex when a site does not yet have active patients or enrollment is slow or challenging due to a rare patient population or very complicated and specific enrollment criteria. In these situations, judicious site management can facilitate more timely and cost-effective deployment of resources, and can bridge gaps in CRA assignment for monitoring tasks as well as improve continuity and consistency of site communications related to turnover, scheduling conflicts, or travel challenges and interruptions.
These factors, including clinical technologies, study design, enrollment, and resourcing challenges add to the growing complexity of clinical trials and necessitate taking a broader view of site needs with additional experts who can support sites when they need assistance and oversight. Our solution is to assess projects that may benefit, due to their complexity, with a separation of the monitoring and site management responsibilities.
Enter the site manager
This intersection of dynamic forces has sharpened focus on the site manager, an expert with a specific purpose to streamline communication with and between sites, and to focus on a specific, critical aspect of a clinical trial such as site activation, recruitment, technical support, or cell and gene therapy logistics. The site manager can provide consistent, expert site management across as many as 20-40 sites when:
- A primary point of contact is needed to rapidly respond to site needs when CRAs are conducting onsite monitoring visits or traveling.
- Disease complexity/rarity requires specific expertise to support a large number of sites.
- The monitoring strategy relies heavily on onsite and/or remote monitoring due to data volume or cadence of data collection.
- The site management strategy is more critical than onsite monitoring due to a low volume of patients and data at any given site.
As the primary site contact, the site manager provides routine site management, as well as specialized expertise in areas such as rare disease, pediatrics, or cell and gene therapy. The function works best when the role is filled by an experienced CRA with strong organizational and communication skills, and a creative, problem-solving mindset. The site manager’s role is particularly important when all sites need to be promptly alerted to critical new information, re-trained and/or reminded of protocol nuances, contacted to collect, collate, and analyze information and feedback from sites, or to assess trial challenges and issues in a consistent manner.
Although site management is generally a non-traveling position, the site manager should be adept at convening site meetings and group calls, and should be a prioritization master adept at handling vendors, Good Clinical Practice (GCP) training, patient recruitment, and other essential facets of site operations.
When to use a site manager?
Not every trial needs a site manager. Nevertheless, deploying a site manager can be crucial to a trial’s success in in a variety of circumstances ranging from small to large, as in the following variable scenarios:
- An ultra-rare disease study with seven sites in three countries, with each site expected to enroll as few as one or two patients over three years. Those patients are treated with a cell therapy and followed closely for two years, then followed on a quarterly basis for the next eight years. In that situation, a site manager with experience in a similar rare disease or cell therapy can assist all the sites in identifying patients and coordinating logistics related to screening, apheresis, cell manufacturing, and chain of custody.
- Larger trials, such as an acute skin infection study with 40 sites in the US, with each site expected to enroll 10 patients over two years. The patients are treated for seven days, leaving some sites with no active patients for months at a time. To ensure consistency, a site manager with experience in acute skin infections can perform routine site management for all sites, while serving as a reference point for onsite CRAs conducting SDR/SDV.
- Large global studies such as a Parkinson’s trial with 60 sites in the US and 40 in Europe. Each site is expected to enroll 20 patients over nine months, with patients referred from physicians’ practices as well as from Parkinson’s advocacy groups. The patients are treated for 18 months with visits every four weeks, then every eight weeks. A site manager with experience in advocacy group relations and community-based outreach can manage patient recruitment across all sites, convene meetings to share best practices, and coordinate study-wide planning, outreach, and patient engagement.
- Technology-enabled research, as in a 30-site, US-based trial of a wearable device to monitor patients with bipolar disorder, with each site expected to enroll 10 patients over 18 months, and a two-year treatment period plus two years of follow-up. For this type of trial, a site manager experienced in complex rater trainings, vendor system training, and device training can manage activation across sites, providing consistency as well as a single point of contact for sponsors. The site manager may also provide guidance to assigned CRAs performing onsite SDR/SDV or fielding device- or vendor-specific questions.
Reaping the benefits of using a site manager
Site managers can deliver numerous benefits to studies and sponsors, particularly in terms of facilitating communication and effective and efficient resource utilization. They can be especially valuable in streamlining information-gathering for improved decision-making through assessment and management of risks, challenges, and potential solutions. An important role of the site manager is to ensure robust communication and information sharing with the CRAs responsible for monitoring. The connection between site management and monitoring is vital for maintaining optimal monitoring oversight.
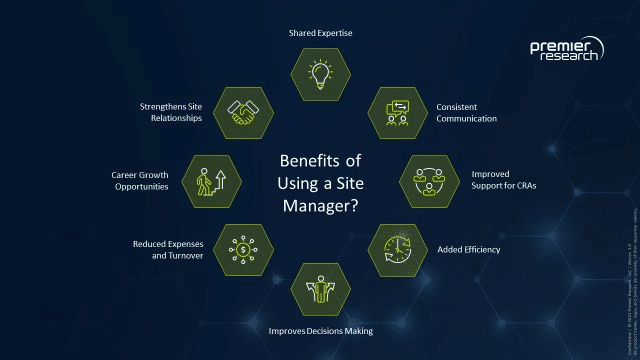
To that end, clinical trial management systems (CTMS) with strong communication interfacing and action item modules can help keep CRAs and site managers on the same page. Site managers can use a CTMS to “trigger” CRAs to perform interim monitoring visits (IMVs) when there is sufficient workload, risks, or challenges requiring an onsite intervention. With dashboards designed to manage information pertaining to site activation, recruitment/enrollment, SDR/SDV backlog, protocol deviations, and other action items, today’s CTMS technologies can drive efficiency and streamline communication across sites.
For more information about Premier’s approach to site management and monitoring or to speak with an expert concerning the specifics of your study, feel free to contact us.

 Webinar
Webinar 


 Perspectives Blog
Perspectives Blog 