Last Updated: August 27, 2025, 4 pm UTC
Long-term follow-up (LTFU) is a defining feature of oncology and cell and gene therapy (CGT) trials, where post-treatment monitoring often spans 2 to 15 years. These extended timelines allow regulators to assess long-term safety and efficacy from detecting delayed adverse events to understanding vector persistence but they also present significant operational and logistical hurdles.
In traditional brick-and-mortar (B&M) models, maintaining patient engagement and consistent data collection over a decade or more is challenging. Sites may be asked to keep their doors open for a handful of patients, incurring substantial costs and administrative burden. Patients, meanwhile, often face travel requirements, scheduling conflicts, and a disconnect from the study team once their active treatment ends. These factors contribute to attrition rates that can undermine the statistical power of the study and, potentially, cause a delay to regulatory milestones.
Sponsors are increasingly exploring decentralized approaches that streamline the LTFU process, reduce burden on both patients and sites, and ensure high-quality data over the long haul.
Why LTFU Is So Critical
In oncology, long-term monitoring can span two years or more; in CGT, FDA guidance calls for follow-up of up to 15 years to ensure patient safety. During this period, developers track:
- Late-onset toxicities or secondary malignancies
- Durability of treatment response and survival outcomes
- Quality-of-life indicators
- Laboratory and imaging results to monitor for disease progression
Without robust follow-up, these datasets may be incomplete, hindering regulatory review and limiting the therapy’s labeling potential. Gaps can also impact patient care — if late effects aren’t identified, providers may miss opportunities for early intervention.
The Retention Challenge
Dropout during LTFU is common. Reasons include:
- Logistical barriers – Distance to sites, transportation issues, or relocation
- Financial strain – Travel costs, time off work, and other expenses
- Perceived low value – Patients may feel visits are unnecessary once they feel well
- Life changes – Moves, changes in insurance, or shifting priorities over many years
Compounding the issue, patients often receive little ongoing communication from their trial team after active treatment, making the relationship easier to let go.
The Decentralized Advantage
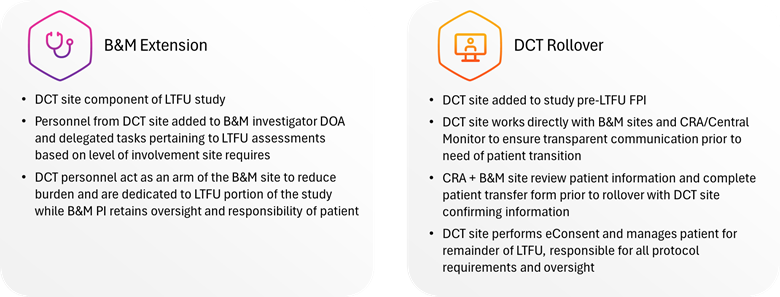
Decentralized long-term follow-up replaces or supplements the traditional site-based model with a centralized “virtual site” that manages all patient contact, data collection, and regulatory documentation. Depending on study needs, this can be implemented in two ways (see Figure 1):
- Full DCT Roll-Over – At the end of the core protocol, patients are reconsented and transferred to a single virtual site that manages all follow-up. Communication occurs via the patient’s preferred method, including text, phone, video, or email. Medical record collection is handled remotely, and data flows directly into the EDC.
- Hybrid Model – For trials where closing all B&M sites isn’t feasible, decentralized personnel can support existing sites, handling outreach, scheduling, and data collection while sites retain clinical oversight.
Both approaches can be customized, allowing sponsors to balance efficiency with site relationships.
Figure 1: Decentralized follow-up reduces burden, increases flexibility, and enables long-term compliance across large and diverse patient populations.
Benefits for Sponsors and Patients
- Stronger Retention
Virtual follow-up eliminates the need for in-person visits, reducing travel and scheduling burden. Regular, personalized communication from quarterly check-ins to life-event tracking, - Cost Efficiency
Maintaining dozens of low-volume sites for years can be prohibitively expensive. A single DCT site reduces IRB renewals, staff turnover risk, and overhead, with the ability to scale resources up or down as enrollment changes. - Faster, Cleaner Data
Passive medical record collection from local providers allows for timely safety reporting while minimizing patient burden. Centralized teams use integrated technology platforms for near real-time updates, minimizing lag between events and data capture. - Built-In Flexibility and Compliance
FDA guidance for CGT LTFU explicitly allows local providers to perform required exams and labs, opening the door to decentralized collection. This flexibility reduces patient burden while ensuring adherence to regulatory requirements. - Geographic Inclusivity
Because follow-up can be conducted from anywhere, patients who relocate for work, school, or family reasons can remain in the study, reducing early terminations due to life changes.
What It Looks Like Operationally
In practice, the decentralized model is supported by a dedicated long-term follow-up team often including coordinators, patient engagement and retention associates, and central PI/sub-I personnel. This team:
- Maintains regular contact with each patient in their preferred format
- Tracks life events and contact changes to prevent lost to follow-up terminations
- Coordinates with local healthcare providers for record retrieval
- Ensures consistent messaging across the patient population
- Meets, or exceeds, all protocol-specified data requirements
Sponsors can choose whether to transition all patients at once, roll over patients site-by-site as they complete active follow-up, or keep some sites open while centralizing others.
Early Planning is Essential
The most successful LTFU strategies are designed into the protocol from the outset. Early planning enables:
- Clear site expectations – Sites know from the start whether follow-up will be handled centrally or locally, avoiding misunderstandings later.
- Smooth transitions – Defined rollover processes and communication plans prevent patient confusion and delays.
- Technology integration – Patient tracking systems, eConsent tools, and data capture platforms can be aligned from day one.
- Regulatory alignment – Protocol language can reflect decentralized processes, reducing the need for mid-study amendments.
When Full DCT Isn’t Possible
Even if a full centralized rollover isn’t feasible, decentralized elements can still be deployed to strengthen LTFU. Adding a virtual team to site delegation logs allows them to assist with outreach, data collection, and retention strategies, providing valuable support without replacing the site entirely.
The Bigger Picture
Decentralized LTFU isn’t just about efficiency, it’s about sustainability. Oncology and CGT programs are pushing trial durations longer than ever, and operational models need to evolve accordingly. Sponsors that adopt patient-centric, flexible, and scalable follow-up approaches can protect the integrity of their data, improve the patient experience, and reduce operational risk over the long term.
In an era when every data point counts, retaining patients and maintaining data quality over years, not just months, can be the difference between a delayed program and a successful regulatory submission.
Premier’s deep expertise in decentralized LTFU studies can offer a more efficient, flexible, and patient-centered approach. To learn how to leverage this model in your next study, contact us.
ABOUT PREMIER RESEARCH:
Premier Research, a global clinical research, product development, and consulting company, is dedicated to helping innovators transform life-changing ideas and breakthrough science into new medical treatments. We offer strategic solutions across the entire development lifecycle, from pre-clinical through commercialization, specializing in smart study design and full-service clinical trial management.
Leveraging technology and therapeutic expertise, we deliver clean, conclusive data with a focus on reducing development timelines, securing access to the right patients, and effectively navigating global regulations to ensure submission-ready results. As an organization that puts patients first, we pride ourselves on helping customers answer the unmet needs of patients across a broad range of medical conditions. Visit premier-research.com.

 Webinar
Webinar 


 Perspectives Blog
Perspectives Blog 