Last Updated: January 6, 2026, 4 pm UTC
Advances in scientific knowledge and growth in the cell and gene therapy space have led to a new and exciting era of medicine for patients, as well as a new motivation for regulators to provide clear, efficient pathways for product developers. These therapies frequently target diseases in patients that are rare (affecting fewer than 200,000 people in the US), serious, and representing an unmet medical need. As a result, programs to develop them often meet the criteria for expedited product development and review and can benefit greatly from early interactions with the FDA to avoid unnecessary and potentially costly preclinical or other preparatory studies.
The INTERACT (INitial Targeted Engagement for Regulatory Advice on CBER ProducTs) meeting offers sponsors of biologics (industry and investigators) an avenue for engagement with CBER in an early development discussion.
Background: The Advancement of Cell and Gene Therapies
Scientific innovation has been at the forefront of navigating the challenges posed by the unprecedented public health emergency brought about by COVID-19. This innovation also continues to thrive across the pharmaceutical industry, as demonstrated by shifts in focus in oncology, rare diseases, and other areas of high unmet need that require new, more complex approaches to drug development.
Rapid scientific advances have put the cell and gene therapy field at the forefront of biomedical research. Gene therapy involves modifying or introducing genes into a patient’s body with the goal of treating, preventing, or curing a disease, while cell therapy requires transferring cells with relevant function into a patient. Both have tremendously evolved in recent years. In chimeric antigen receptor T-cell (CAR-T) therapy, cells are genetically modified before being (re)introduced into the patient, representing the intersection between gene and cell therapy.
Recent expansion, advances, and discoveries in cell and gene therapy initiatives have led to a new and exciting era of medicine for patients and are set to lead to even more important breakthroughs. A 2020 PhRMA report on the cell and gene therapy pipeline found 362 therapies in a range of stages in clinical development by biopharmaceutical companies in the United States.
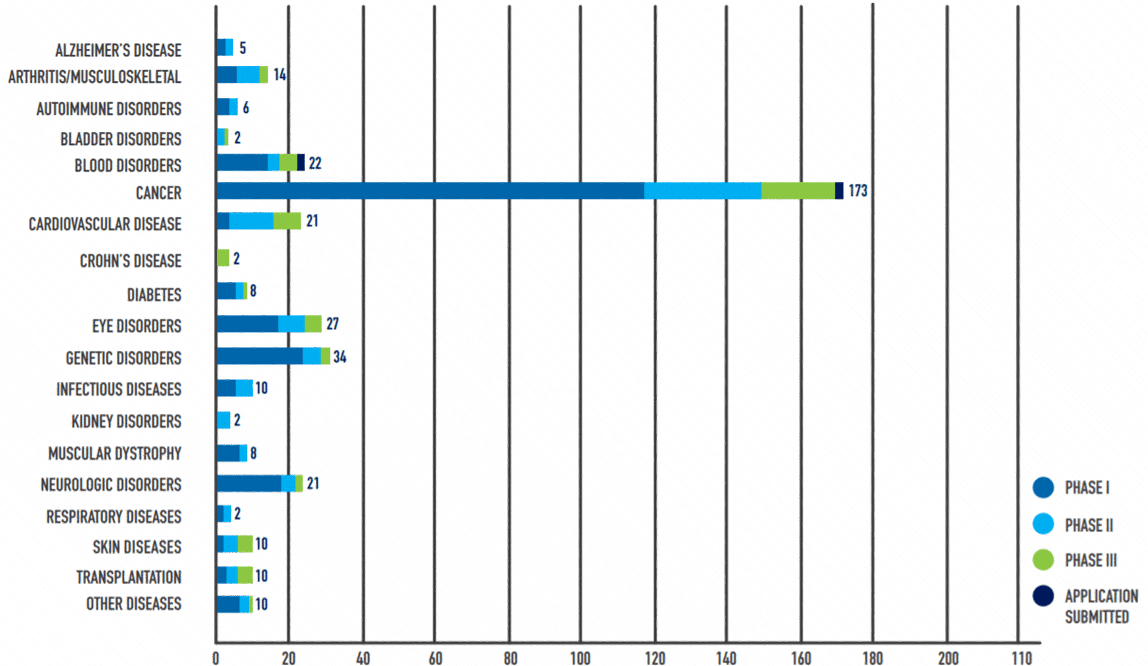
Note: Some medicines are in more than one category.
Source: Medicines in Development | 2020 Update: Cell and Gene Therapy, PhRMA
As a result of this area’s rapid growth and complexity, unique challenges to product development confront sponsors early on. Therefore, interacting with CBER’s Office of Tissues and Advanced Therapies (OTAT) at the earliest stage of development is critical to ensuring the proposed development program addresses the unique and complex regulatory requirements for cell and gene therapies.
The Purpose of INTERACT Meetings
INTERACT meetings are intended for sponsors with an identified novel product (or product‑derivation strategy) with unique safety profile challenges related to complex manufacturing technologies, innovative devices, or new testing methodologies. The meeting has several goals:
- Discuss issues critical to early product development
- Obtain a preliminary, informal consultation with CBER prior to a Pre-IND meeting
- Obtain initial, nonbinding advice regarding chemistry, manufacturing, and controls (CMC), pharmacology and toxicology, and/or the clinical aspects of a development program
- Discuss new delivery devices, early product development, and/or pre‑clinical proof‑of concept
- Identify critical development issues or deficiencies to address
Scope and Topics: INTERACT vs Pre-IND Meetings
INTERACT
- CMC: Innovative technologies, product manufacturing, reagents, starting materials, critical product components, novel delivery device qualification, and complex software issues
- Pharmacology/toxicology: Design of proof-of-concept studies, pilot safety/biodistributions studies, selection of animal models, and innovative testing strategies, products, and delivery
- First-in-human trial
- Devices: Complex software issues and analytical requirements for innovative blood screening devices
- Challenges due to unknown safety profiles
Pre-IND
- Scope and design of definitive animal trials and clinical trials
- Topics that fall under the CBER Advanced Technologies Team (CATT)
- Product selection
- Previous regulatory advice on a similar product and indication
- Toxicology study design considerations for IND-enabling studies
- Pre-reviews of completed studies
- Preclinical testing plans (without preliminary data from pilot studies)
- Certain clinical study protocol design considerations that fall under pre-IND meetings
Anatomy of a Successful INTERACT Meeting
An INTERACT meeting is not a forum for a sponsor to present development options to the FDA for discussion. The objective of the meeting is to obtain feedback on a proposed pre-defined program.
First, a sponsor must submit a meeting request and package together (no more than 50 pages) in the following format:
- Cover letter: Identification of the specific CBER office for consultation and a clear indication that the package is a request for an INTERACT meeting
- Product information: Product description, dosage form, and route of administration
- Proposed indication: Description of disease or condition
- Product development: History and future plans
- Purpose and objectives of the meeting
- Meeting information: Suggested dates and times for the meeting, along with a list of participants with their titles and affiliations
- List of questions: Questions grouped by topic (CMC, pharmacology/toxicology, clinical) and summary of data to support discussion grouped by topic and question
A sponsor must provide concise detail in the meeting package and ask specific, targeted questions so that the FDA can provide meaningful guidance. Remember, only those questions listed in the package will be discussed during the INTERACT meeting.
The FDA typically responds to the meeting request within 21 calendar days and schedules the meeting 90 calendar days from receipt.
It is up to the sponsor to identify concerning development issues, as the FDA will not provide unsolicited information. A sponsor should highlight those sticky issues in the meeting package then prioritize them for discussion at the beginning of the INTERACT meeting to allow enough time for feedback.
Each participant must be prepared, as every second counts in a one-hour meeting. When the FDA provides preliminary meeting comments (typically one day prior to the meeting), the team should review the comments and determine the order of the meeting questions, who will lead the meeting, and which team members will speak on each question. Lastly, a ‘dry-run’ meeting should be held to practice what each person plans to say during the actual meeting.
After the meeting, the FDA will not provide meeting minutes, so a sponsor should designate a team member whose sole role is to take notes. Documenting the feedback is important so that you can incorporate it into subsequent meetings with the FDA as your development program proceeds to the Pre-IND stage.
The FDA can help in the development of novel innovative products, and seeking early guidance, particularly when working with cell and gene therapy products, is invaluable when navigating complex or challenging preliminary product development strategies. An INTERACT meeting can provide crucial insight on how to accelerate your program and save time and money in the process.
Premier Consulting, a division of Premier Research’s, frequent regulatory interactions with CBER and OTAT, our extensive drug development experience, and our multidisciplinary teams uniquely position us to navigate clients through complex, challenging cell and gene therapy product development programs. Contact us to find out how we can help you take the important first step of gaining early communication with the FDA via an INTERACT meeting.

 Webinar
Webinar 

 Perspectives Blog
Perspectives Blog 