Skin diseases are the fourth leading cause of health afflictions, affecting nearly 900 million people worldwide.1,2 At the same time, there has been a remarkable increase in dermatology drug development activity in recent years. For conditions such as atopic dermatitis, psoriasis, and melanoma, there are hundreds of ongoing or planned clinical trials.3 In this new and extremely competitive era, there is more pressure than ever to optimize your clinical development strategy and planning for successful study recruitment, retention, and implementation.
In this blog post, based on our recent webinar, we explore critical study design and operational success factors for this new wave of dermatology clinical trials.
Addressing unmet needs in dermatology
There are more than 3,000 common and rare skin diseases, and the need for safe and effective therapeutic solutions for most of these diseases remains largely unaddressed. While our understanding of the etiology, epidemiology, and pathophysiology of dermatologic conditions has grown rapidly in recent years, much of the development success to date is in a small subset of diseases with multiple approved therapeutics (see Figure 1).
Figure 1. Top seven dermatologic indications with the highest number of approved drugs
| Indication | Psoriasis | Bacterial Infections | Acne | Melanoma | Atopic Dermatitis | Rosacea | Actinic Keratosis |
| # of FDA Approved Drugs | 13 | 12 | 9 | 9 | 6 | 4 | 3 |
Over the past 10 years, however, the number of drugs approved by the FDA for rare skin diseases has risen, particularly for skin-related cancers (see Figure 2).4 There has also been a flurry of research activity, with many development projects involving innovative, targeted medicinal products driven by our advanced understanding of certain skin diseases’ biology.
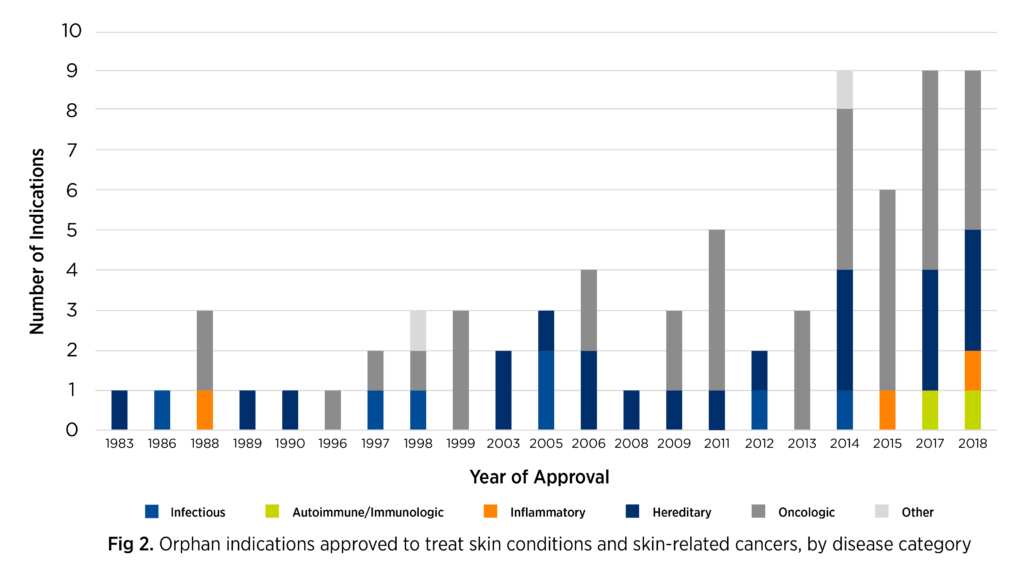
Adapted from Karas L, et al. J Am Acad Dermatol. 2019;867-877.
In this intense dermatology development space, it’s essential to carefully evaluate study design and operations to gain a competitive edge and increase your probability of success.
Study design considerations
Given global circumstances, 2020 has been a year of fierce learning and acute adjustments. Many of the study design changes implemented in 2020 are likely to be incorporated into future clinical trials as they have proven to be safe, time-saving, cost-effective, and/or more convenient for patients or site personnel.
When drafting a protocol, it is critical to identify all potential elements of risk and develop contingency plans for addressing those risks. An intense focus on patient safety is a powerful framework for decision-making when it comes to study design. The first set of questions you should be asking is how to safely recruit, enroll, and follow up with patients and how to ensure patients feel safe about participating in a clinical trial. In Figure 3, we review some important protocol considerations.
Figure 3. Top 2020 considerations for dermatology clinical trial protocols
| Consent | Is it possible to implement e-consent or remote paper consent? |
| Study visits | How long should on-site visits be? Can some visits be remote via telemedicine or home health? Which visits are essential for preserving data integrity, quality, and study endpoints? |
| Enrollment and retention | What strategies can be implemented at the study and site level to improve recruitment and engagement? |
| Study procedures and assessments | Which efficacy assessments will be used? How will investigations and raters be trained? Is there a need for biopsies, are they mandatory or optional, and how often will they occur (less will be more for study feasibility)? What laboratory tests are important, and what is the optimal frequency for testing? Can certain procedures and/or assessments be performed off-site by a home nurse? What assessments can be completed via telemedicine and which are critical to be conducted in person? |
| Study drug | Key strategic question: What is the nature and behavior of the investigational medicinal product (IMP),and how does it align with the biology of the targeted disease?Operational questions of the year:
|
| Data Quality | Can source document verification be reduced or performed remotely? Would a combination of central(risk-based)and remote monitoring work for the study? How will efforts to maintain study data integrity be documented? |
Incorporating patient feedback on the protocol is becoming essential and will provide a competitive edge, especially in indications where clinical trial activity is brisk or eligible patient populations are small.
Operational considerations
Based on our clinical trial experience, we have identified the following key operational considerations that contribute to the success of dermatology studies:
- Accelerating start-up. Working with a contract research organization that has an established network of investigators can help to streamline start-up. At Premier Research, we know our sites and may be able to waive site qualification visits, saving time during initial site engagement. In addition, factors such as licensing of patient-reported outcome questionnaires and translation of documents can have a significant impact on study timelines. Getting started on these rate-limiting factors as early as possible can help keep studies on track. Using sites with which Premier has an existing relationship, we can reference certain site documents from previous studies to shorten the onboarding time. Using site contract templates is the most time-saving part of getting sites started up quickly and can take weeks off the study.
- Ensuring rater consistency. Rater consistency is invaluable to the success of dermatology studies and their associated regulatory submissions. Having a process in place to create and implement rater training and tracking raters from visit to visit is critical.
- Reducing source data verification (SDV). Minimizing the need for SDV can help to reduce duplication of work, time on site, and study costs. Utilizing Premier’s risk-based monitoring system, data can be analyzed in real-time once entered into the electronic case report form. With this system, 100 percent of the data is reviewed, but SDV is reduced, while still ensuring data quality.
Key takeaway
Our surveillance of dermatology’s new and emerging landscape indicates increasingly intense clinical research effort in this space as sponsors race to address unmet needs for patients living with skin diseases. To learn more about optimizing clinical development strategy and planning to accelerate the path the regulatory approval, view our webinar: A New Era in Dermatology: Study Design, Regulatory Strategies, and Patient Participation.
1 Seth D, Cheldize K, Brown D, Freeman EF. Global burden of skin disease: inequities and innovations. Curr Dermatol Rep. 2017;6(3):204-210.
2 World Health Organization. Recognizing neglected skin diseases: WHO publishes pictorial training guide. Available at https://www.who.int/neglected_diseases/news/WHO-publishes-pictorial-training-guide-on-neglected-skin-disease/en/.
3 GlobalData, Dermatology, Relevant Clinical Trials. Accessed September 2, 2020.
4 Karas L, et al. The impact of the Orphan Drug Act on Food and Drug Administration-approved therapies for rare skin diseases and skin-related cancers. J Am Acad Dermatol. 2019;81(3):867-877.