Last Updated: July 22, 2025, 9 am UTC
For decades, the Alzheimer’s disease (AD) treatment landscape remained largely static, focused on symptom management rather than disease modification. But in recent years, the field has entered a transformational period. With the emergence of new biomarkers, approval of novel anti-amyloid therapies and a pipeline rich in disease-modifying approaches, we are witnessing the emergence of a new era in AD research and drug development.
From first-in-class therapies to evolving trial designs and biomarker-based diagnostics, here’s how the landscape is shifting—and what sponsors need to know to stay ahead.
From Symptom Management to Disease Modification
Historically, AD treatments consisted of cholinesterase inhibitors like donepezil and NMDA antagonists such as memantine, providing modest symptom relief without altering disease progression. That changed with the approvals of aducanumab, lecanemab, and donanemab—monoclonal antibodies that target amyloid beta.
While aducanumab was withdrawn in 2024 following controversy over its clinical efficacy and uptake,i lecanemab (Leqembi) and donanemab (Kisunla) have set a new standard. Both received U.S. FDA approval and demonstrated meaningful reductions in cognitive decline among early-stage patients. In clinical trials, over a period of 18 months:
- Lecanemab slowed the rate of cognitive decline by 27% compared to placeboii
- Donanemab slowed cognitive and functional decline by 35-36% compared to placeboiii
The clinical impact is equally promising: individuals treated with these agents may remain independent nearly 10 months longer than those on placebo. Specifically, a recent publication calculated that a typical person with very mild symptoms could expect to live independently for another 29 months without treatment, 39 months with lecanemab, and 37 months with donanemab. These outcomes underscore a critical shift—early-stage intervention is now the central focus of AD drug development.
Barriers to Widespread Use
Despite their promise, anti-amyloid therapies face significant real-world challenges that currently limit uptake:
- IV Infusion Burdeniv: Lecanemab and donanemab must be administered in the clinic every 2–4 weeks. Subcutaneous injectables are in development and may eventually reduce patient burden and expand access.
- Access and Diagnosis: A confirmed AD diagnosis—typically via PET imaging or cerebrospinal fluid biomarkers—is required. This narrows the eligible population and adds logistical complexity, especially in underserved regions. However, many countries are pending regulatory approval.
- Safety Considerations: Both drugs carry black box warnings for amyloid-related imaging abnormalities (ARIA), including cerebral edema (ARIA-E) and hemorrhages (ARIA-H).v Close MRI monitoring is essential, further taxing already limited clinical infrastructure.
As the field looks ahead, emerging therapies and trial designs must address these barriers to ensure broader patient benefit.
A Pipeline Powered by Innovation
According to the analysis by Cummings et al., the 2025 Alzheimer’s drug pipeline includes (see Figure 1):vi
- 182 clinical trials assessing 138 drugs
- Roughly 70% of investigational drugs are disease-modifying therapies (DMTs)
- 53% are small molecules
- 47% are biologics
Figure 1: 2025 AD Drug Development Pipeline
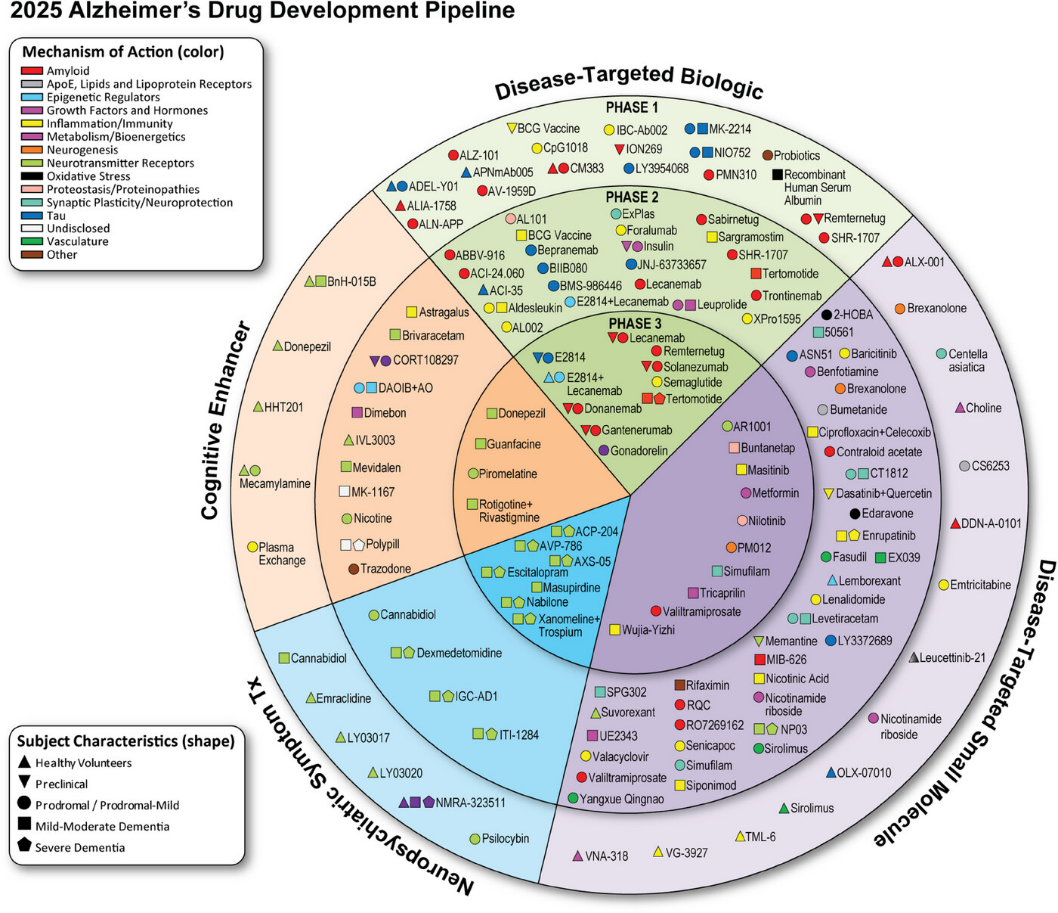
A growing number of trials are investigating combination therapies, which aim to amplify therapeutic impact by pairing anti-amyloid treatments with agents that target tau pathology, neuroinflammation, or synaptic dysfunction. These multi-drug approaches may unlock more substantial disease modification—possibly reducing progression by 50–70%.vii
However, combination trials bring their own complexities, including the need for robust safety monitoring, optimized stratification, and innovative designs that accommodate evolving standard-of-care treatments.
Evolving Regulatory Expectations
The U.S. FDA is actively shaping the development environment for early-stage AD treatments.viii Recent draft guidance emphasizes:
- Earlier intervention, focusing on stages 1–3 (from preclinical AD to mild cognitive impairment)
- Use of biomarkers—especially amyloid and tau—as surrogate endpoints
- Greater reliance on biomarker-confirmed diagnoses
- Movement away from traditional scales like ADAS-Cog toward more sensitive composite endpoints
The agency also encourages early engagement with sponsors on trial design and regulatory strategy—particularly when pursuing accelerated approval pathways.
This evolving framework aligns with the growing recognition that AD is a biologically definable condition long before clinical symptoms emerge, and that early intervention may be critical for achieving meaningful outcomes.
The Rise of Biomarker-Driven Diagnosis
Reliable detection of preclinical Alzheimer’s is foundational for earlier intervention and trial success (see Figure 2). Several tools are shaping this landscape:
- Amyloid PET Imaging: The gold standard for amyloid detection, but costly and limited in availability.ix
- CSF Biomarkers: Validated measures of Aβ42/40 ratio and tau proteins, though invasive and often met with patient resistance.x
- Tau PET: Expensive and not yet widely accessible but emerging as a powerful tool for risk stratification.xi
- Blood-Based Biomarkers: A potential game-changer, these non-invasive markers are under active investigation with the first diagnostic blood test approved in May 2025. While not yet standard, they may dramatically expand screening and enrollment capabilities.xiixiii
Both FDA and EMA require validated assays for trial inclusion, adding complexity but reinforcing scientific rigor.
Figure 2: Biomarker-Driven Diagnosis in Preclinical AD

The Challenge of Preclinical Recruitment
One of the greatest challenges facing early-stage AD trials is identifying and enrolling preclinical participants—individuals with no cognitive symptoms but with biomarker evidence of disease. While enriched populations, such as individuals with APOE4 positivity, can improve recruitment efficiency, they also limit generalizability and may skew trial outcomes.
Moreover, many of the tools required for early diagnosis—such as PET scans or lumbar punctures—are not widely available, adding further obstacles to trial execution.
To overcome these hurdles, sponsors must take a multi-pronged approach that includes:
- Investing in broad-based screening infrastructure
- Utilizing digital tools to identify at-risk populations
- Building diverse trial networks to ensure representative enrollment
- Supporting trial networks in expanding their community outreach and education efforts
Translating Progress into Impact
The Alzheimer’s field is evolving at a pace unmatched in its history. With new disease-modifying therapies on the market and a deep pipeline of innovative agents—including combination therapies and next-generation biomarkers—the opportunity to transform the trajectory of AD has never been greater.
Yet, realizing this potential will require careful navigation of regulatory expectations, operational complexities, and scientific frontiers. By staying aligned with emerging trends and leveraging early strategic planning, sponsors can be part of the next chapter in Alzheimer’s drug development—one that holds real promise for patients and families alike.
Ready to move your Alzheimer’s program forward? Our CNS team has the scientific and therapeutic insight and operational know-how to help you design efficient, effective trials in this rapidly evolving space. Contact us to learn more.
ABOUT PREMIER RESEARCH:
Premier Research, a global clinical research, product development, and consulting company, is dedicated to helping innovators transform life-changing ideas and breakthrough science into new medical treatments. We offer strategic solutions across the entire development lifecycle, from pre-clinical through commercialization, specializing in smart study design and full-service clinical trial management.
Leveraging technology and therapeutic expertise, we deliver clean, conclusive data with a focus on reducing development timelines, securing access to the right patients, and effectively navigating global regulations to ensure submission-ready results.
As an organization that puts patients first, we pride ourselves on helping customers answer the unmet needs of patients across a broad range of medical conditions. Visit premier-research.com.
REFERENCES:
[I] Biogen Press Release. Biogen to Realign Resources for Alzheimer’s Disease Franchise. January 31, 2024. https://investors.biogen.com/news-releases/news-release-details/biogen-realign-resources-alzheimers-disease-franchise
[II] The Lancet. Lecanemab for Alzheimer’s disease: tempering hype and hope. Lancet. 2022 Dec 3;400(10367):1899. doi: 10.1016/S0140-6736(22)02480-1. PMID: 36463893.
[III] Klein EG, Schroeder K, Wessels AM, Phipps A, Japha M, Schilling T, Zimmer JA. How donanemab data address the coverage with evidence development questions. Alzheimers Dement. 2024 Apr;20(4):3127-3140. doi: 10.1002/alz.13700. Epub 2024 Feb 7. PMID: 38323738; PMCID: PMC11032520.
[IV] https://stanfordhealthcare.org/campaigns/lecanemab.html
[V] Roytman M, Mashriqi F, Al-Tawil K, Schulz PE, Zaharchuk G, Benzinger TLS, Franceschi AM. Amyloid-Related Imaging Abnormalities: An Update. AJR Am J Roentgenol. 2023 Apr;220(4):562-574. doi: 10.2214/AJR.22.28461. Epub 2022 Nov 2. PMID: 36321981.
[VI] Cummings, J., Bain, L. J., Shihabuddin, L., Pixley, H. T., Zhong, K., Nehme, J., Li, Z., Ma, L., & Friedhoff, L. T. (2024). 2025 Alzheimer’s disease drug development pipeline: Continued growth in anti-amyloid, anti-tau, and neuroinflammation approaches. Alzheimer’s & Dementia: Translational Research & Clinical Interventions, 10, e70098. https://doi.org/10.1002/trc2.70098
[VII] Boxer AL, Sperling R. Accelerating Alzheimer’s therapeutic development: The past and future of clinical trials. Cell. 2023 Oct 26;186(22):4757-4772. doi: 10.1016/j.cell.2023.09.023. Epub 2023 Oct 16. PMID: 37848035; PMCID: PMC10625460.
[VIII] U.S. Food and Drug Administration. (2024). Early Alzheimer’s disease: Developing drugs for treatment: Draft guidance for industry. https://www.fda.gov/media/110903/download
[IX] Chapleau M, Iaccarino L, Soleimani-Meigooni D, Rabinovici GD. The Role of Amyloid PET in Imaging Neurodegenerative Disorders: A Review. J Nucl Med. 2022 Jun;63(Suppl 1):13S-19S. doi: 10.2967/jnumed.121.263195. PMID: 35649652; PMCID: PMC9165727.
[X] Amft M, Ortner M, Eichenlaub U, Goldhardt O, Diehl-Schmid J, Hedderich DM, Yakushev I, Grimmer T. The cerebrospinal fluid biomarker ratio Aβ42/40 identifies amyloid positron emission tomography positivity better than Aβ42 alone in a heterogeneous memory clinic cohort. Alzheimers Res Ther. 2022 Apr 26;14(1):60. doi: 10.1186/s13195-022-01003-w. PMID: 35473631; PMCID: PMC9044878.
[XI] Groot C, Villeneuve S, Smith R, Hansson O, Ossenkoppele R. Tau PET Imaging in Neurodegenerative Disorders. J Nucl Med. 2022 Jun;63(Suppl 1):20S-26S. doi: 10.2967/jnumed.121.263196. PMID: 35649647.
[XIII] McGettigan S, Nolan Y, Ghosh S, O’Mahony D. The emerging role of blood biomarkers in diagnosis and treatment of Alzheimer’s disease. Eur Geriatr Med. 2023 Oct;14(5):913-917. doi: 10.1007/s41999-023-00847-1. PMID: 37648817.
[XIII] Bouwman FH, Frisoni GB, Johnson SC, Chen X, Engelborghs S, Ikeuchi T, Paquet C, Ritchie C, Bozeat S, Quevenco FC, Teunissen C. Clinical application of CSF biomarkers for Alzheimer’s disease: From rationale to ratios. Alzheimers Dement (Amst). 2022 Apr 27;14(1):e12314. doi: 10.1002/dad2.12314. PMID: 35496374; PMCID: PMC9044123.

 Webinar
Webinar 


 Perspectives Blog
Perspectives Blog 