Last Updated: January 6, 2026, 7 am UTC
The traditional entry point for clinical research organization (CRO) engagement is delivery of the first clinical research study. However, companies with limited internal resources may benefit from engaging with a CRO as early as possible to leverage the full breadth of experience that a CRO can bring to the partnership. The right CRO will bring the integrated regulatory, clinical, and medical expertise necessary to streamline development, ensure compliance, and could accelerate commercialization.
In this blog, we explore pivotal time points for engaging a CRO, highlighting how an experienced partner can contribute at different stages of development.
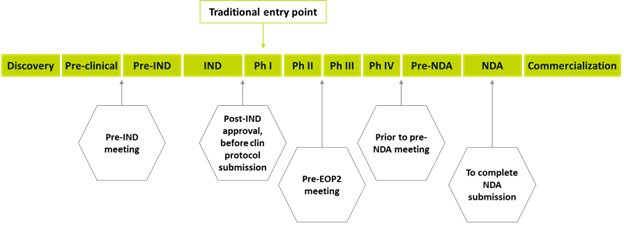
Figure 1. Potential time points for CRO engagement
Time point 1: Before the pre-IND meeting
An optimal entry point is prior to the pre-investigational new drug (IND) meeting, which will allow the sponsor to take full advantage of the breadth of the CRO’s experience. For example, the CRO can conduct a gap analysis and strategic assessment of each element of the development plan, from pharmacology and pharmacokinetics/pharmacodynamics (PK/PD) to nonclinical; chemistry, manufacturing, and controls;, and quality. The CRO can also provide guidance on the first-in-human (FIH) study design and protocol and assist with the technical writing, publishing, and submission of the briefing package.
The benefits of engaging with a CRO before the pre-IND meeting include:
- Early identification and mitigation of potential risks
- Streamlined regulatory interactions that pave the way for a smoother IND approval process
- Establishment of a strong scientific foundation for subsequent phases of development
Time point 2: Post-IND approval and before clinical protocol submission
If brought in at the approval stage of the FIH study, the CRO can provide a feasibility and operational delivery assessment, including analysis of enrollment rates based on disease prevalence and historical data, with a country/site mix to support the proposed timelines. Engagement at this stage allows sponsors to also benefit from the CRO’s experience to ensure the development plan is geared towards a successful end of phase 2 (EOP2) meeting to gain agreement with the expected phase 3 study and overall new drug application (NDA) strategy.
This is also a stage where the CRO can begin formulating a strategic delivery plan by evaluating team members from all functional departments with the skillset and desire to follow the asset throughout development. This approach promotes team stability to maintain institutional knowledge and enhance collaboration. From a CRO perspective, teams working in this model often have increased engagement and lower turnover.
Partnering with a CRO after IND approval offers the following advantages:
- Increased operational efficiency and resource utilization
- Improved likelihood of meeting clinical milestones and regulatory expectations
- Stronger partnership between the CRO and sponsor teams, fostering better communication and program continuity
Time point 3: Pre-EOP2 meeting
The EoP2 meeting focuses on fine-tuning the development program to ensure readiness for late-stage clinical trials and eventual market approval. If engaged to support at this critical juncture, the CRO will conduct a gap assessment and then create and work through a mitigation plan to bridge identified gaps. Examples of work that a CRO might recommend or implement include:
- Revisions to the proposed phase 3 protocol to ensure it supports labeling goals
- A target product profile (TPP) that offers a structured template summarizing minimal and preferred therapeutic attributes and crucial drug labeling details
- A proposed draft label
If not already considered, the CRO may also recommend expedited programs such as Fast Track, Breakthrough Designation, Accelerated Approval, or Priority Review. Where applicable, the CRO may also evaluate the feasibility of orphan drug designation, which offers market exclusivity, tax credits, and fee waivers.
Key benefits of engaging with a CRO at this stage include:
- Proactive risk management to address potential regulatory concerns before pivotal trials
- Alignment of clinical development with commercial objectives
- Opportunity to expedite development timelines with special regulatory pathways
Time point 4: Prior to pre-NDA meeting
If brought in to support the pre-NDA meeting, the CRO will focus on reviewing all previously-completed gap assessments and prior communications with regulatory agencies. This review is critical for ensuring that clear approaches have been defined for addressing all gaps and deficiencies. Having a structured NDA plan with timelines for completing all activities, including authoring of modules and engaging with regulatory agencies in the months leading up to submission, is fundamental for success.
Of note, this is one of the most difficult time points at which to engage a CRO partner for the first time. It is essential for the sponsor to allocate ample time for the CRO to get up to speed. To facilitate full onboarding, the developer must have an organized repository of all previous engagements with regulatory agencies, as well as all internal considerations and discussion that led to key decisions that informed the development path.
At this stage, CRO engagement provides:
- Expert guidance on finalizing the NDA submission package
- Assurance that all regulatory expectations have been met, reducing the risk of review delays
- Much-needed support to the internal team during a resource-intensive phase
Time point 5: NDA publishing and submission
A final entry point for CRO engagement is to complete publishing and submission of the NDA package. While this is an indispensable deliverable requiring technical expertise, it is a discrete activity that does not leverage the breadth of the CRO’s experience. Rather than contributing to the overall development plan, the CRO is primarily focused on delivering a distinct technical service.
Nevertheless, the CRO partner adds value by providing:
- Just-in-time access to specialized technical skills and resources
- Efficient and compliant submission processes
- Time for internal teams to focus on responding to regulatory queries and preparing for product launch
A strategic partner at every point
Moving an asset through development is a multifaceted process and partnering early with a CRO that has relevant experience, expertise, and resources can optimize the process. The ability to flexibly scale up or scale down experienced resources across all stages of the process to supplement or guide a sponsor’s internal team increases the probability of success. In addition, leveraging CRO processes provides stability and continuity if there is unexpected turnover with sponsor resources.
Understanding when and how a CRO can support asset development from program strategy to commercial launch enables companies to maximize the benefits of outsourcing to an experienced partner who serves as an agile extension of the internal team (see Figure 2).
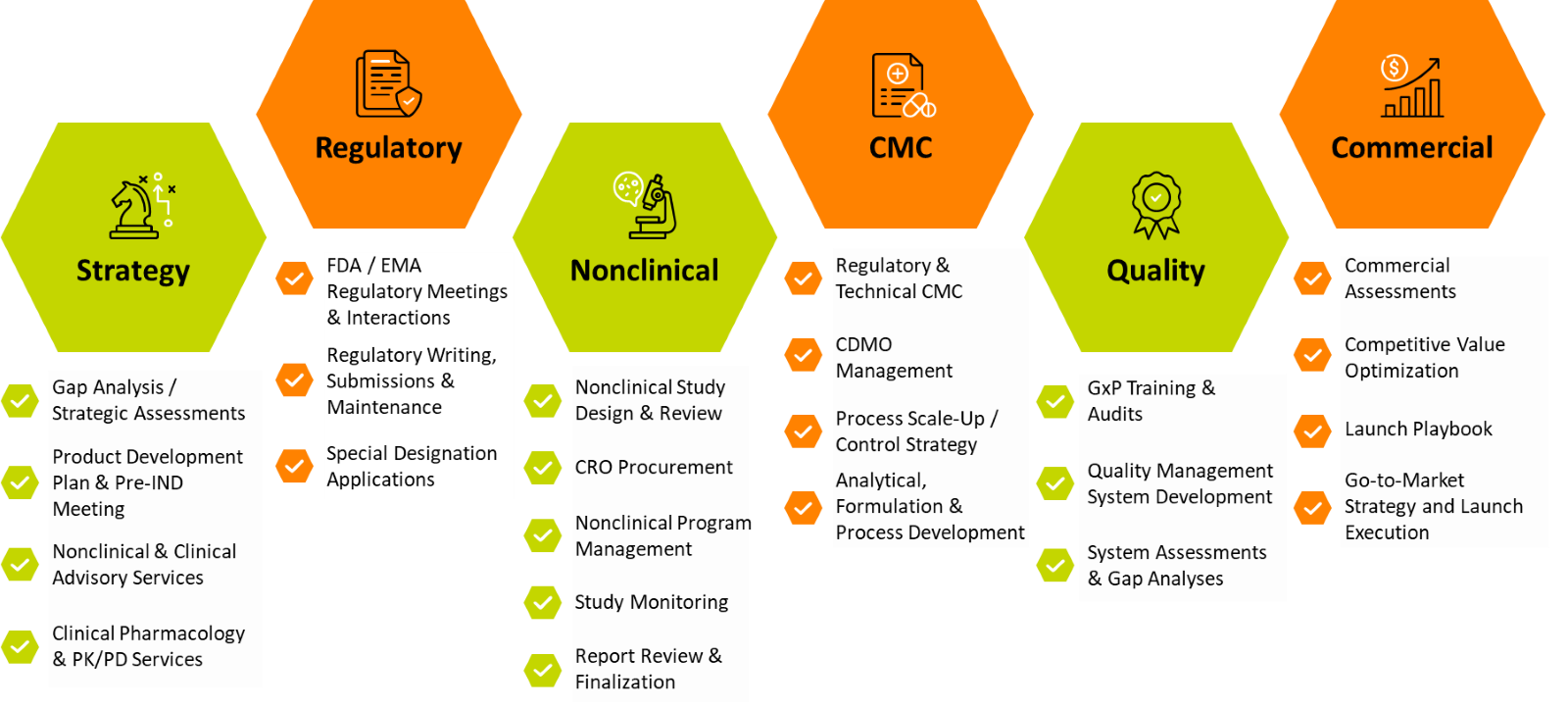
Figure 2. How Premier Research supports asset development
To learn more about how early and effective engagement with a CRO that can support the entire development timeline, from clinical trials to commercialization, contact us.
About Premier Research:
Premier Research, a global clinical research, product development, and consulting company, is dedicated to helping innovators transform life-changing ideas and breakthrough science into new medical treatments. We offer strategic solutions across the entire development lifecycle, from pre-clinical through commercialization, specializing in smart study design and full-service clinical trial management.
Leveraging technology and therapeutic expertise, we deliver clean, conclusive data with a focus on reducing development timelines, securing access to the right patients, and effectively navigating global regulations to ensure submission-ready results.
As an organization that puts patients first, we pride ourselves on helping customers answer the unmet needs of patients across a broad range of medical conditions. Visit premier-research.com.

 Webinar
Webinar 


 Perspectives Blog
Perspectives Blog 