Last Updated: January 6, 2026, 7 am UTC
In the realm of clinical trials, patient involvement is not just a nicety—it’s a necessity. The patient voice has become central to the success of clinical trials, shifting the focus from traditional stakeholders like healthcare providers and regulatory bodies to those who are most affected: the patients themselves.
“We owe it to the patients to bring forward the patient voice. We owe it to the physicians, to the agencies, to all of the stakeholders involved to elevate every patient that could be potentially impacted by that disease.”
The inclusion of patient insights can illuminate real-world challenges and preferences, making clinical trials not only more patient-friendly but also more effective in yielding relevant data. This approach ensures that trials are designed with an understanding of patient needs, ultimately leading to better outcomes and more successful treatments. Patient advocacy groups (PAGs) have emerged as trusted intermediaries between researchers and patient communities, providing valuable insights into patient concerns, experiences, and priorities.
This blog delves into the critical role PAGs play in amplifying the patient voice in clinical trials and highlights key strategies for effectively engaging and building relationships with these groups.
Harnessing patient insights for clinical and commercial success
PAGs provide an avenue to essential patient perspectives, not just for clinical development but also for the commercial success of medical products. Understanding the patient journey—from diagnosis to treatment—provides a comprehensive view that informs both trial design and market strategies.
PAGs can support:
- Patient-centric trial design: PAGs contribute to designing trials that are more aligned with patient needs and preferences.
- Identifying pain points: They help identify what aspects of a trial or treatment are most meaningful to patients, such as managing fatigue or pain levels.
- Enhancing market readiness: Insights from patients can guide the development of products that meet the market’s needs, ensuring successful adoption once a product is approved.
- Accelerating drug development and regulatory engagement: Regulatory agencies recognize the value of patient input. PAGs can advocate for patient-centric endpoints and influence policy changes that benefit the broader patient community.
- Fostering long-term engagement beyond trials: Collaboration extends beyond clinical trials, as patient groups can support post-market surveillance, real-world evidence generation, and ongoing treatment adherence initiatives.
“Really getting that rich information to inform on endpoints from these types of groups not only is critical to the trial itself, but also meaningful for when you bring that asset to market.”
Engaging effectively with patient advocacy groups
Building effective relationships with PAGs in clinical research is essential. To build robust partnerships with PAGs, consider the following key factors (see Figure 1).
Figure 1: Keys to Successful PAG Partnerships
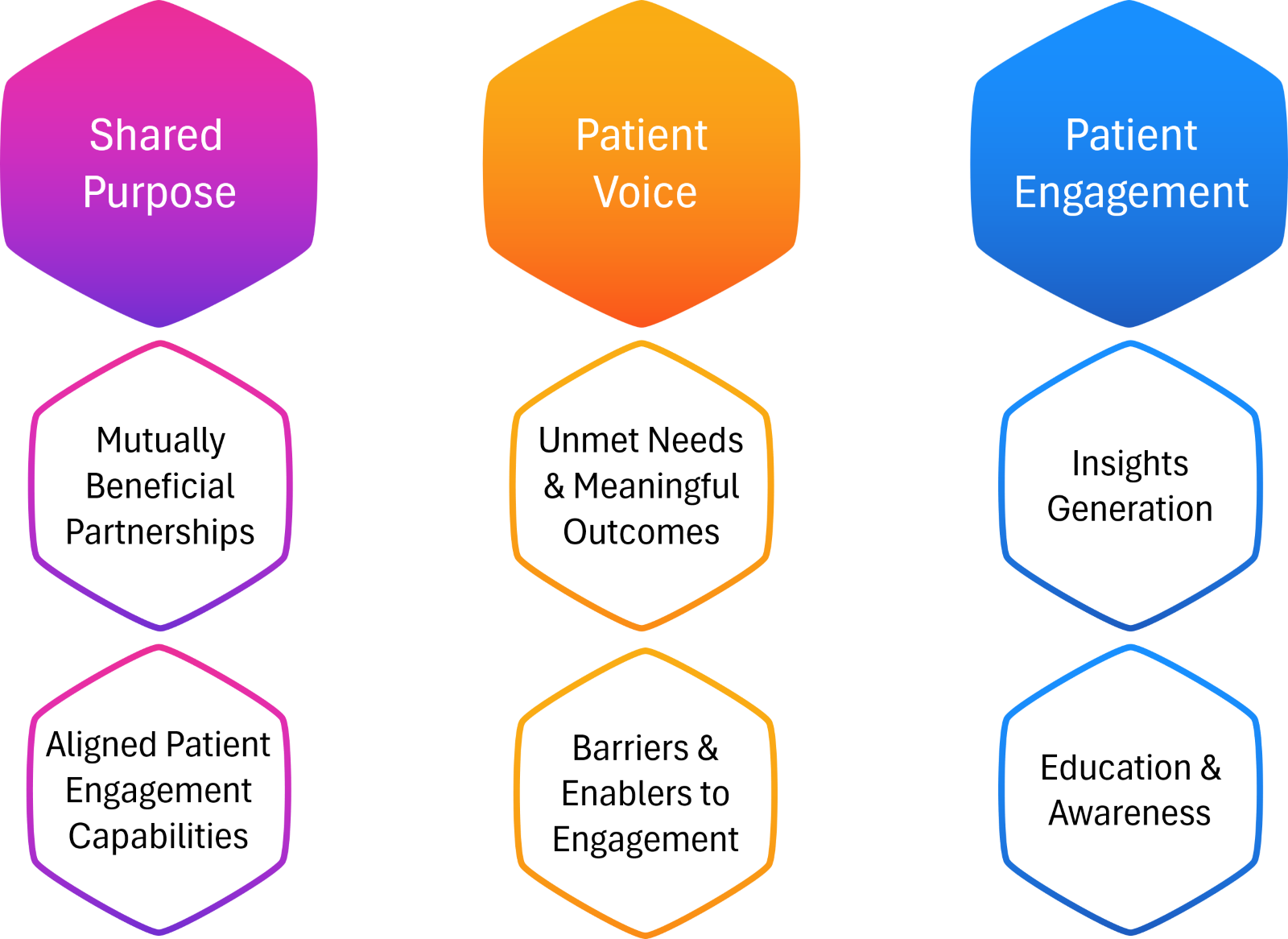
- Align goals: Establish clear alignment with the patient advocacy group’s mission and vision.
- Understand capabilities: Assess the group’s ability to engage their network and contribute effectively to trial goals.
- Support education and awareness efforts: Equip PAGs with accurate, plain-language resources to help educate their communities about clinical trials.
- Engage continuously: Foster an ongoing relationship rather than a one-off interaction, ensuring long-term support and collaboration. Consider sharing findings from current studies, including challenges or unexpected results, and discussing future research opportunities. Build trust by being transparent about study outcomes.
“Really trying to cultivate those robust relationships throughout the longevity and the lifetime of that relationship is key.”
The imperative of diversity in medical research
While recent administration changes in the US have resulted in the FDA removing its guidance on Diversity Action Plans from its website, diversity in clinical trials is still essential for the validity and applicability of research findings. Historical oversights, such as the exclusion of women from trials in the past, have highlighted the need for inclusive research practices (see Figure 2).
Figure 2: Inclusion of Women in Clinical Trials: A Historical Overview of Scientific, Ethical, and Legal Issuesi

PAGs are essential in helping to bridge gaps between researchers and underrepresented communities. Their deep connections with diverse patient populations allow them to:
- Improve outreach and awareness: PAGs engage with communities that have been traditionally underrepresented in research, including racial and ethnic minorities, rural populations, and those with socioeconomic barriers. Through culturally tailored education and outreach, they help dispel misconceptions and increase trial awareness.
- Address barriers to participation: Many underserved groups face logistical, financial, and trust-related challenges that hinder trial participation. Advocacy groups work with sponsors to identify and mitigate these barriers by advocating for travel support, childcare assistance, and decentralized trial options.
- Build trust and cultural competency: Historical distrust in medical research has led to low participation rates among certain populations. PAGs, as trusted voices, help foster confidence in the research process by ensuring transparency, engaging in dialogue, and advocating for ethical study designs.
- Advocate for inclusive trial design: By providing input on study protocols, PAGs help ensure eligibility criteria are not overly restrictive and that trials reflect the real-world patient population. They also push for the inclusion of patient-reported outcomes that are meaningful to diverse communities.
“We owe it to all patients to ensure inclusion and appropriate representation in medical research.”
Keeping the patient voice in focus
In clinical research, patient involvement, insights, and diversity are key to success. By prioritizing these elements, trials not only become more inclusive and representative but also more effective in developing treatments that truly meet patient needs. As the industry continues to evolve, engaging and growing relationships with PAGs will be critical to keep the patient voice at the forefront, ensuring that research and development efforts are both scientifically rigorous and socially responsible.
“Ensuring that every patient sees themselves in the data that we pull forward is so meaningful.”
For support with your clinical development program, contact us.
About Premier Research:
Premier Research, a global clinical research, product development, and consulting company, is dedicated to helping innovators transform life-changing ideas and breakthrough science into new medical treatments. We offer strategic solutions across the entire development lifecycle, from pre-clinical through commercialization, specializing in smart study design and full-service clinical trial management.
Leveraging technology and therapeutic expertise, we deliver clean, conclusive data with a focus on reducing development timelines, securing access to the right patients, and effectively navigating global regulations to ensure submission-ready results.
As an organization that puts patients first, we pride ourselves on helping customers answer the unmet needs of patients across a broad range of medical conditions. Visit premier-research.com.

 Webinar
Webinar 


 Perspectives Blog
Perspectives Blog 