Pediatrics
Bridging Innovation and Compassion to Bring New Treatments to Pediatric Patients
Leverage Our Pediatric Clinical Development Expertise
Age Makes a Difference in Medical Care.
Adults react differently to medicine compared to children and adolescents. Still, most medicines given to children have been tested only in adults, raising numerous concerns about efficacy and safety. That’s changing, and it has become clear that pediatric trials require a special approach.
To succeed in pediatrics requires a deep understanding of childhood diseases, from the familiar to the extremely rare. And it requires an appreciation of the various stages of childhood development from birth through late adolescence.
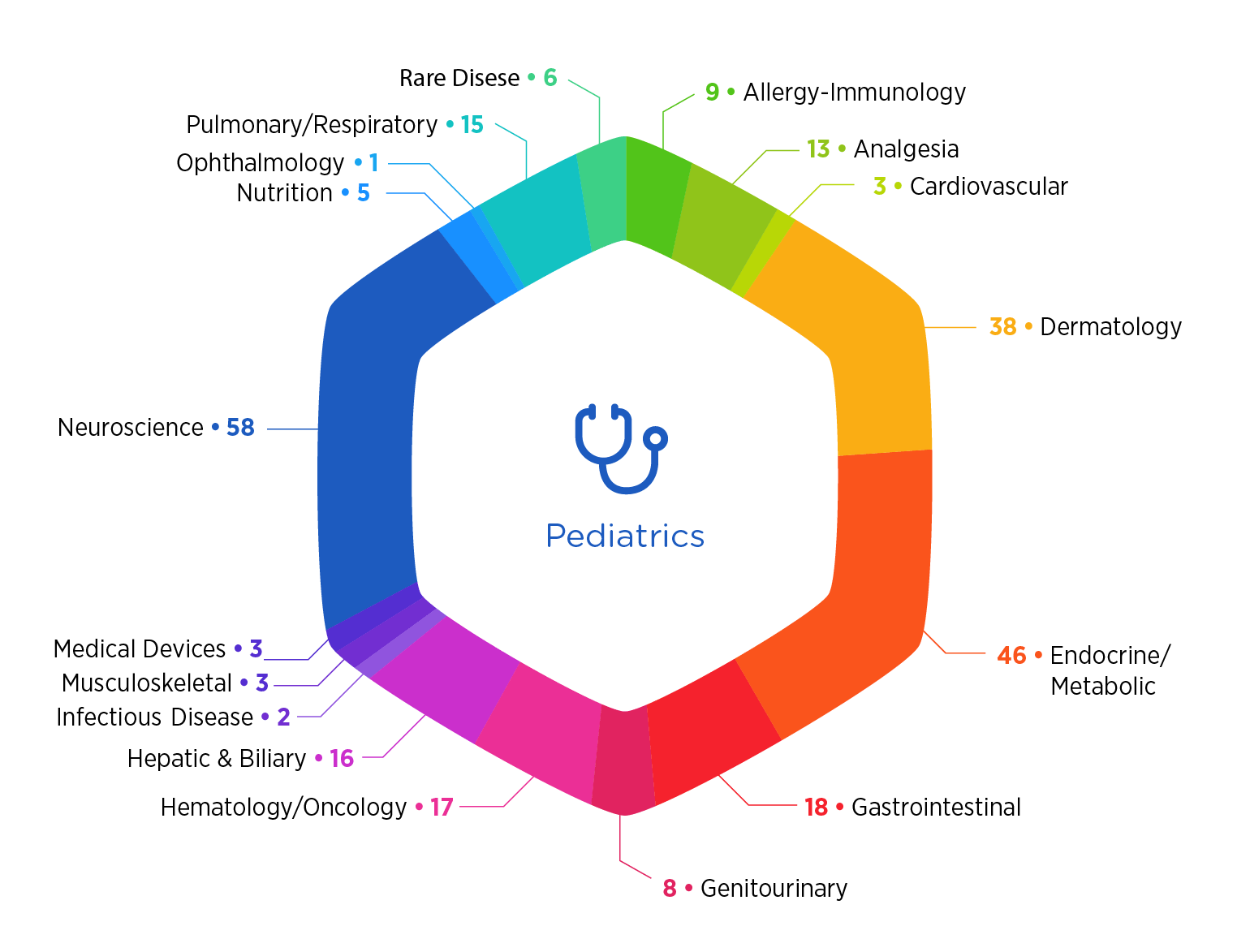
Why Choose Premier
- More than 250 pediatric studies completed over the past five years
- Specialized training is delivered to our operational team members, including a custom pediatric research training certification program
- Six years as sole coordinating center for the Best Pharmaceuticals for Children Act
- Project teams benefit from strategic insights of trained pediatric physicians with years of clinical and R&D experience
- Considerable depth of regulatory expertise, including frequent support of Pediatric Research Equity Act requests and waivers
Agility and knowledge at your fingertips
Resources
Perspectives Blogs
Understanding Recent Regulatory Changes for Pediatric Oncology Trials
Of the 1.7 million new cases of cancer in the U.S.

Articles
5 Things to Know About the FDA RACE for Children Act
The Research to Accelerate Cures and Equity (RACE) for Children Act aims to improve and expand treatment options for pediatric cancer patients by mandating that all new adult oncology drugs also be tested in children when the molecular targets are relevant to a particular childhood cancer.

Perspectives Blogs
Food for Thought: The Power of Decentralized Clinical Trials in Accelerating Infant Formula Studies
Infant formula is an essential food product that serves as the sole source of nutrition for many babies in North America and Europe during their first year of life, supporting health, growth, and development.1,2 More than a year since the shutdown of the largest infant formula plant in the US in February 2022, the country is still feeling the shortage.
Meet the Experts
Pediatrics

Angi Robinson
Senior Vice President
Specialty Areas (Rare Disease, Pediatrics, Cell & Gene Therapy)

Adam Bloomfield, M.D.
Vice President
General Medicine, Rare Disease & Pediatrics, Medical Affairs

Paulla Dennis
Executive Director
Program Strategy, Rare Disease, Pediatrics, Cell & Gene Therapy