Observational, Registry, & Post-Marketing Studies
Bridging Clinical Development and Real-World Application
Full-Service and Consulting Solutions for Strategic Observational, Registry, and Post-Marketing Studies
In today’s evolving research landscape, real-world data (RWD) and real-world evidence (RWE) have become indispensable in understanding how therapies perform beyond controlled clinical settings. Global regulators have started to embrace the use of real-world, unstructured data alongside traditional randomized control trial data, a paradigm shift that sets the stage for new. This trend is paving the way for more efficient and creative approaches to drug development and commercialization, especially for developers of oncology, rare disease, and emergency use products.
Premier Research leverages its clinical expertise and advanced data analytics to design and execute observational and post-marketing studies that provide actionable insights into treatment effectiveness, safety, and patient outcomes in real-world scenarios.
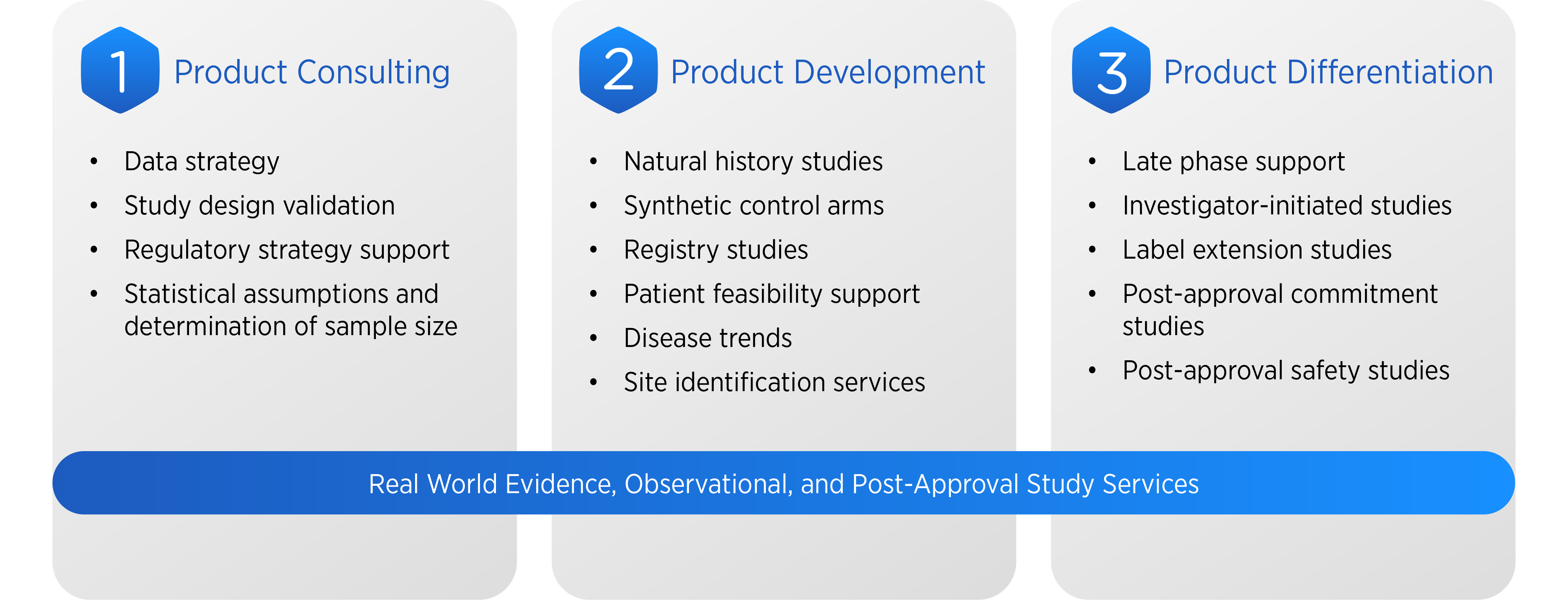
Our Integrated Services are Tailored to Support:
- Registry Studies: Capturing data on treatment patterns, outcomes, and resource utilization without intervention, providing insights into real-world effectiveness.
- Natural History Studies: Systematically collecting data on disease progression to inform clinical trial design, especially crucial in rare diseases with limited existing data.
- Post-Marketing Surveillance: Monitoring long-term safety and effectiveness of approved therapies to meet regulatory requirements and inform clinical practice.
Comprehensive Support
Well-designed studies shorten timelines, lower costs, improve the impact of research investments, and get therapies to patients faster. Leveraging a cross-functional team with deep regulatory, nonclinical, clinical, commercial, and therapeutic expertise, we optimize data collection, analysis, and submission.
- Improved Stakeholder Engagement: We help you provide payers, providers, and patients with evidence of real-world effectiveness and safety to support adoption and reimbursement
- Enhanced Development Plan: We utilize real-world insights to validate study design, refine inclusion/exclusion criteria, select meaningful endpoints, and improve protocol feasibility.
- Informed Regulatory Strategy: We strategically generate evidence that complements clinical trial data, aiding in regulatory decision-making processes and submission strategy.
Why Choose Premier?
In a multilateral shift, sponsors, payers, regulators, physicians, and patients are increasingly recognizing the value of real-world data assessment and late phase clinical trials. It is invaluable in bridging the gap from development to commercialization.
We’ll help you optimize your strategy and provide insights for better decision-making across the health care landscape.
- Improve patient recruitment strategies
- Validate study design faster
- Enhance ability to establish data trends to support endpoints
- Ensure real-time access to data
- Deliver successful fast-track regulatory submissions
- Lead successful 505(b)(2) submissions
Premier Research is your trusted partner in generating real-world evidence that drives informed decision-making and accelerates the delivery of effective therapies to patients. Contact Premier today to learn how we can support your clinical development and post-marketing strategies.
Agility and knowledge at your fingertips
Resources

Real-World Data and Real-World Evidence: What Is Their Value as a Synthetic Control Arm?
The Expanding Role of RWE in Rare Studies
resources
Stay ahead of the curve by browsing our extensive library of white papers, case studies, blog posts, and more.