Consulting
Expert Guidance from Concept to Commercialization
Strategic Development Support to Take Your Product to the Next Level
Product development is a journey—complex, fast-moving, and often unpredictable. To navigate from R&D and compliance through IND/IDE and into commercialization, you need more than a plan. You need a strategic partner with deep expertise and the flexibility to adapt when the path changes.
At Premier, we help innovators turn breakthrough science into life-changing solutions. As scientists, strategists, and regulatory experts, we’re driven to bring your vision to life—faster, smarter, and with greater confidence.
Integrated, End-to-End Expertise
Premier provides global product development and regulatory consulting services supported by a cross-functional team of experts across nonclinical and clinical development, CMC, quality, regulatory affairs, regulatory publishing and submissions, trial master file quality management, and market access.
Whether you need full-spectrum guidance or targeted functional support, we offer integrated insights to help you advance through every development milestone—from product concept and regulatory planning to clinical execution and market access.
Strategic Partnership With Purpose
We don’t just consult—we collaborate. Our approach is grounded in science, shaped by experience. We’ll work closely with your team to design and implement product development and regulatory strategies that reduce risk, accelerate progress, and align with your business goals.
From the earliest stages of development through commercialization, we help you approach each step with clarity and confidence. Our consultants understand the scientific and operational challenges you face, and we know how to navigate them. Together, we turn obstacles into opportunities and unknowns into actionable next steps. We meet that complexity with agility, creativity, and executional excellence.
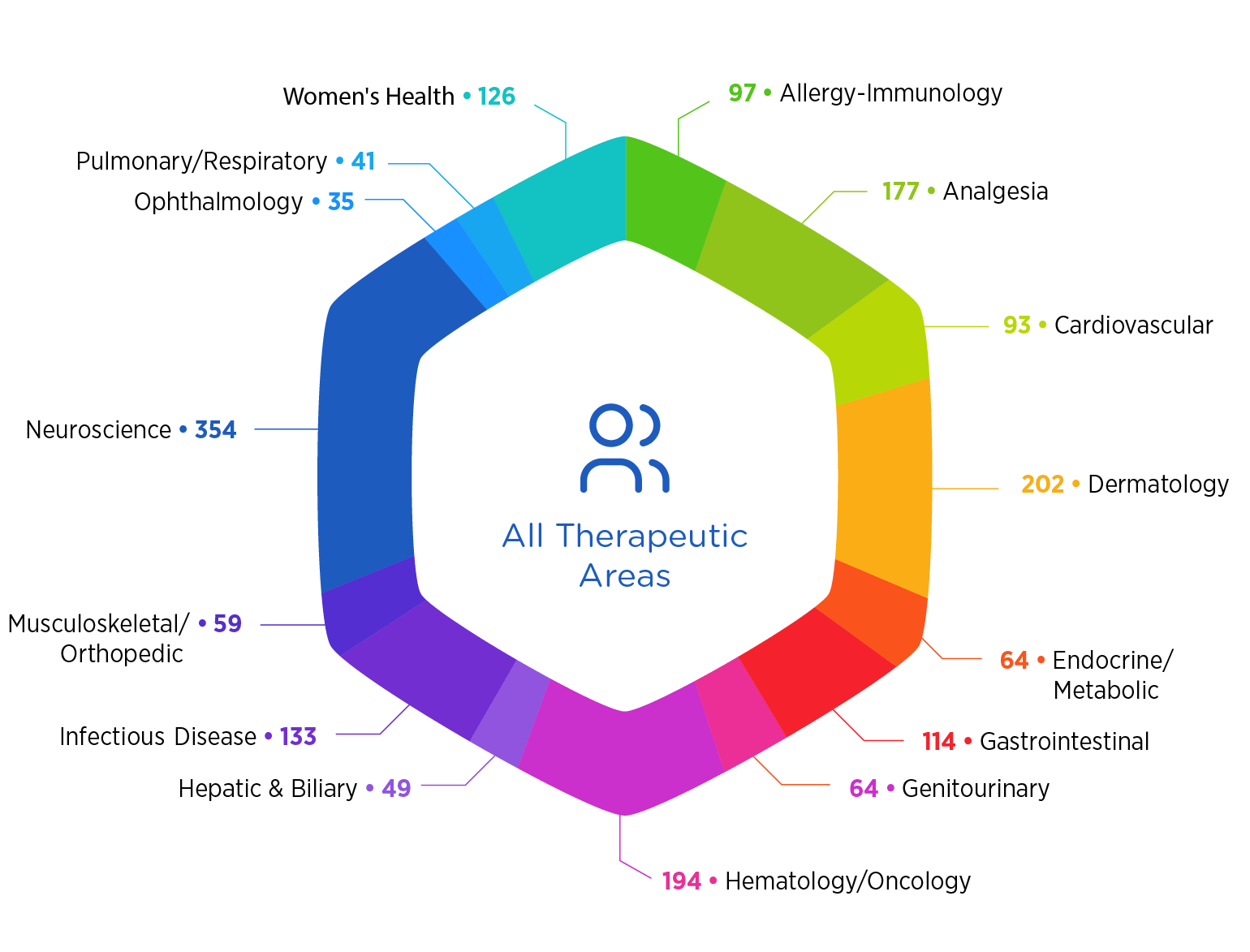
Therapeutic Focus
There’s no substitute for experience. And we have a lot of it. See what we’ve been busy doing for the past five years.
Agility and knowledge at your fingertips
Resources

FDA Eliminates Animal Testing: Impact on Biotechs

The First Step for Streamlined CMC Development: Optimize the Target Product Profile
resources
Stay ahead of the curve by browsing our extensive library of white papers, case studies, blog posts, and more.