Clinical Research & Development
Optimize Every Step of Your Clinical Program
Flexible Full-Service and FSP Solutions to Accelerate Your Development Plan
As therapies grow more advanced, study challenges can compound. Whether you need full-service clinical development or targeted functional expertise, our technology-enabled solutions help you drive efficiency and quality from study design through close-out. Backed by deep scientific insight, regulatory knowledge, and operational precision, we’re here to help you navigate complexity and deliver results.
We understand that no two studies are alike. That’s why we take a collaborative, customized approach—aligning closely with your internal teams to co-develop strategies that are both scalable and adaptive. Our integrated data and analytics capabilities ensure transparency and actionable insights throughout the study lifecycle, enabling real-time decision-making and proactive issue resolution.
Comprehensive Solutions That Deliver
As a full-service contract research organization (CRO) and trusted functional service provider, we bring the flexibility and expertise you need to meet evolving demands. Our teams work seamlessly across functions and geographies, enabling close collaboration, streamlined execution, and the delivery of high-quality, submission-ready data.
From first-in-human studies to global pivotal trials, registry studies to post-marketing surveillance, and customized, global FSP programs, we manage complex projects across all phases, diverse therapeutic areas, modalities, and locations.
Our operational and functional experts are skilled in novel and adaptive study designs, special population engagement, strategic risk-based monitoring strategies, virtual trial implementation, and quality management. We prioritize patient safety and engagement strategies that improve enrollment, retention, and representation—critical to the success and impact of today’s most innovative therapies.
With decades of experience and success to pull from, our team will help define your goals and navigate the challenges of reaching them—all with an unwavering focus on patient safety and a smarter, faster path to approval.
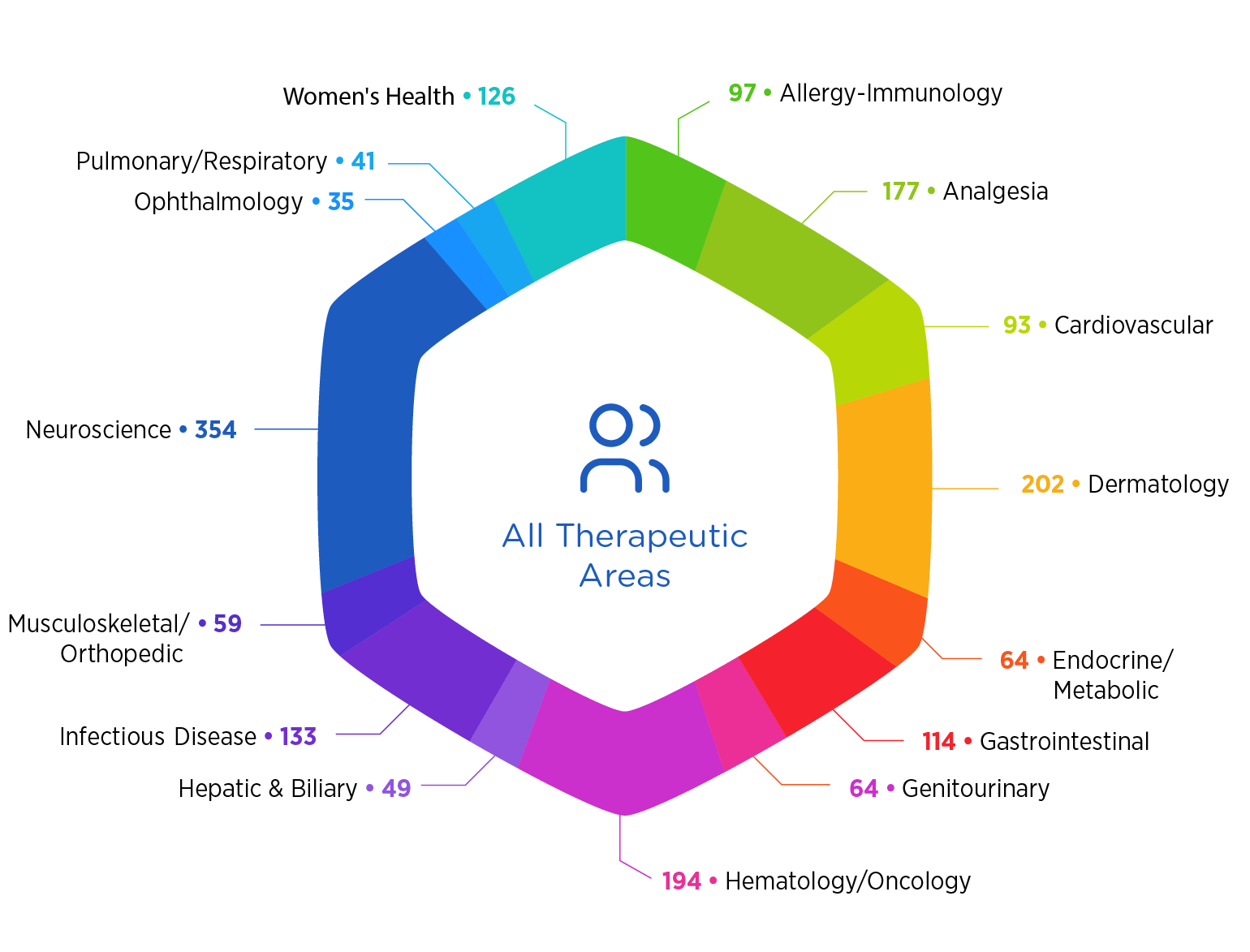
Therapeutic Focus
There’s no substitute for experience. And we have a lot of it. See what we’ve been busy doing for the past five years.
Agility and knowledge at your fingertips
Resources

Full Service or FSP? How to Choose the Right Model for Your Study

Are You Engaging a CRO at the Right Point in Your Drug Development Program?
resources
Stay ahead of the curve by browsing our extensive library of white papers, case studies, blog posts, and more.