Last Updated: October 9, 2024, 10 am UTC
The 21st Century Cures Act (Cures Act)1 signed into United States law in 2016 aims to accelerate medical product development and bring innovations to patients faster through real-world evidence (RWE) to support regulatory decision making. Incorporating real-world data (RWD) and real-world evidence (RWE) can help pharma and biotech companies optimize clinical trial designs and demonstrate that novel medical products are effective in practical application.
While large pharma companies possess the deep resources to launch RWE programs and adjust as needed; small to midsize, specialty pharma and biotech companies must tread more carefully. Their focus is typically on traditional, precedent-based asset development because departure from a well-understood pathway entails risk they can ill afford.
Our three-part blog series outlines an approach for determining whether an RWE program is suitable for a particular development strategy and addresses the extent to which regulators embrace these programs. With proper guidance, small to midsize sponsors can benefit from RWE with minimal risk.
What are RWD and RWE?
The terms RWD and RWE often cause confusion. To clarify, RWD is raw source data related to patient health status and/or healthcare delivery in real-world settings. It is routinely collected from diverse sources (Figure 1). These include:
- Electronic health records
- Administrative claims and billing
- Product or disease registries and surveys
- Pharmacy data
- Mortality data
- Mobile devices
- Biosensors and wearables
- Patient-generated data including social media
- Retail data
The RWD Universe
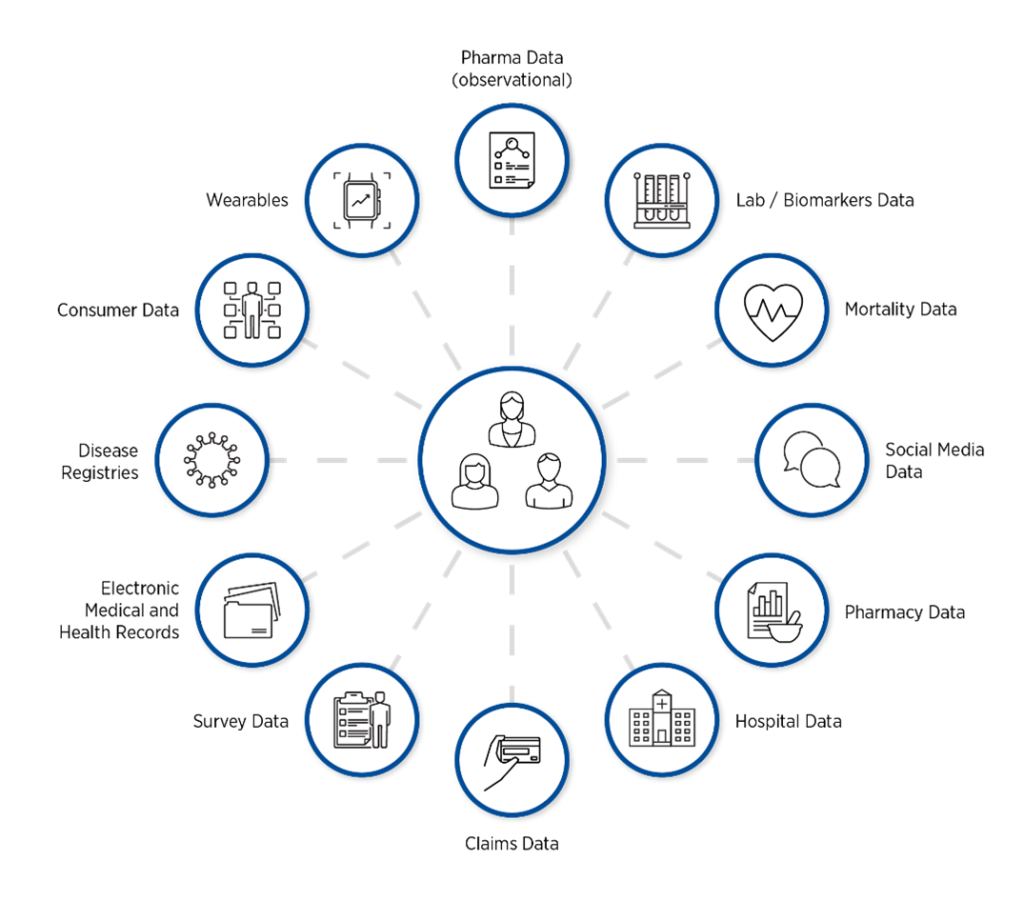
Figure 1. Vast quantities of RWD are available from a multitude of sources.
To appreciate the scale of available data from these sources, consider that it encompasses2:
- 43 million people are currently accruing new data
- 425 million person-years of observation time
- 5.9 billion pharmacy prescriptions
- 7.2 billion unique medical encounters
- 42 million people with at least one laboratory test result
RWE is the synthesis of RWD. Advanced analytical capabilities enable the analysis of de-identified, pooled RWD from multiple sources in the context of other findings to generate evidence. This clinical evidence regarding the real-life usage and potential benefits or risks of a medical product can provide insights about efficacy, safety, or cost different from those obtained during a randomized clinical trial (RCT). The goal of RWE is to provide compelling and complementary data to RCTs so sponsors can hasten the development of innovative treatment approaches, including discovering new indications for approved therapies.
How can RWD be applied to gain insight in clinical development?
Appropriate use of RWD benefits sponsors, payers, and patients across multiple regulatory approval stages (Figure 2). RWE aids in product development and guides regulatory decision-making (label changes), reimbursement decisions, or clinical recommendations. Following regulatory approval, ongoing studies of RWD can help monitor product safety and efficacy.
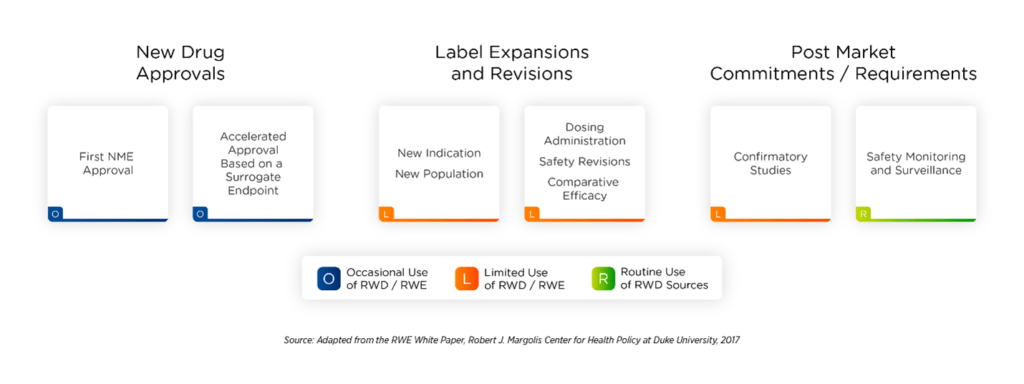
Figure 23. RWD and RWE may be applied from the new drug approval (NDA) stage through post-market safety monitoring.
Depending upon the question to be answered and the types and quality of data available, evidence can be generated to support a variety of clinical trial designs such as large simple trials and pragmatic trials, as well as observational studies. RWD offers sponsors numerous opportunities to generate evidence that provides insight into clinical outcomes and feasibility. A complete understanding of target populations allows sponsors to craft flexible clinical trial protocols to enroll the desired patients on time.
Evaluating outcomes via RWE allows sponsors to better establish the efficacy and safety of a drug or therapy in a real-world setting, to inform both patients and payers. Innovative study designs involving RWE may expedite improved treatments to patients while allowing evidence to be collected at multiple endpoints. Real-time data collection and patient monitoring improve care and clinical decision-making while maximizing value through efficiency and providing further evidence to aid commercialization.
Key Takeaways
RWD is gaining importance as a resource to enhance clinical research.
- RWD encompasses a vast quantity and variety of raw source data related to patient health status and/or healthcare delivery in real-world settings
- RWE is the end result of analyzing RWD
- RWD and RWE find application throughout the clinical development life cycle, from the NDA stage through post-market safety monitoring
- Incorporation of RWE and RWE can help pharma and biotech companies optimize clinical trial designs and demonstrate that novel medical products are effective in practical application
With RWE, every study demands a unique solution. Premier takes a tailored approach that enables even risk-averse sponsors to take advantage of RWD and RWE to support regulatory applications. Click here to learn more about how Premier Research experts can help identify a fit-for-purpose model that helps sponsors make critical decisions about incorporating RWE into their data strategy.
[1] Public Law 114–255 114th Congress An Act. U.S. Government Information, 2016. https://www.govinfo.gov/link/plaw/114/public/255. Accessed May 18, 2022.
[2] Sentinel ARIA Sufficiency and the Regulatory Review Process. Available at: https://www.fda.gov/media/108517/download. Accessed June 20, 2022
[3] Berger M, Daniel G, Frank K, Hernandez A, McClellan M, Okun S, Overhage M, Platt R, Romine M, and Wilson M, 2017a. A framework for regulatory use of realworld evidence. White Paper. The Duke-Margolis Centre for Health Policy.

 Webinar
Webinar 


 Perspectives Blog
Perspectives Blog 