Though gene therapy has been around for decades, it continues to pose extraordinary challenges in the areas of R&D, clinical development, and operation of clinical trials. Sponsors and CROs face shifting operational and regulatory demands amid rapid advances in the science of precision medicine.
In our previous blog post, we discussed site selection, manufacturing, long-term follow-up, and other critical considerations for gene therapy trials at the study design phase. In this follow-up, we’ll continue our case study of a rescue Phase 1/2 gene therapy trial involving localized administration of gene therapy using specialized equipment with a look at the operational challenges of these trials during COVID-19.
The global pandemic has exacerbated the operational challenges intrinsic to gene therapy at the site level, the project team level, and the sponsor level. To ensure that studies continue to move forward, strategies need to be considered not only for operational efficiency and patient centricity but also as contingency planning in the case of a subsequent wave or quarantine (see Figure 1).
Figure 1. Operational strategies during COVID-19
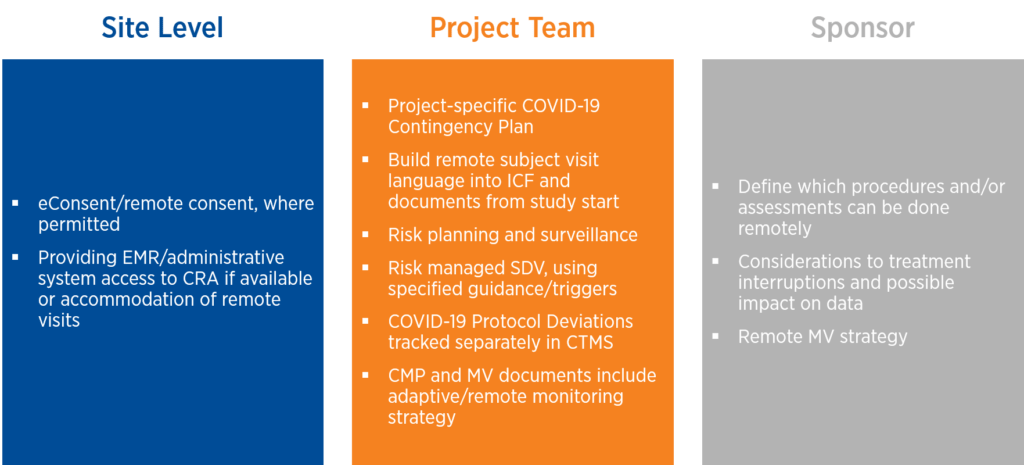
Technology plays a key role, especially now that there are pathways that allow for patient confidentiality while enabling access to the documents needed to conduct source document verification.
At the project team level, it is important to anticipate and plan for challenges that may arise. For example, you may want to consider building remote subject visit language into the informed consent form, and other core documents from the study start to minimize the need for protocol amendments.
At the sponsor level, the challenges stemming from the COVID-19 pandemic have triggered new thinking about which study processes and procedures can be performed off-site by home nurses or remotely via web-based technologies.
Key takeaways
The key lessons learned from this gene therapy case study are to:
- Anticipate all the regulatory questions that may arise in each region or country where the study is to be conducted
- Plan to tackle those questions and get buy-in from investigators
- Understand the impact on patients, especially if the study includes a sham procedure
- Reach out to patient advocacy groups for insight on study design
- Plan for the unexpected by putting into place processes that will allow the study to continue in exigent circumstances without putting the data at risk
Premier Research has conducted 60 gene and cell therapy trials in the past five years in multiple therapeutic areas, including oncology, hematology, neurology, and rare disease. To schedule a conversation with one of our gene therapy experts, click here.