Infant formula is an essential food product that serves as the sole source of nutrition for many babies in North America and Europe during their first year of life, supporting health, growth, and development.1,2 More than a year since the shutdown of the largest infant formula plant in the US in February 2022, the country is still feeling the shortage. To stabilize supply, the FDA exercised temporary enforcement discretion to allow certain covered manufacturers to market infant formulas that may not currently comply with specific agency requirements so long as these manufacturers took specific action to achieve compliance. According to the FDA guidance, Infant Formula Transition Plan for Exercise of Enforcement Discretion, any covered manufacturer wishing to continue marketing their non-exempt infant formula products must submit a New Infant Formula Submission by September 3, 2023, if a clinical study is required.
With covered manufacturers pressing to meet that submission deadline and developers of new infant formulas also seeking to bring their products to market, an increasing number of sponsors are planning infant formula trials. These studies face numerous challenges related to enrollment, including:
- FDA requirement to enroll infants before 14 days of life and to use formula as the sole source of nutrition for the duration of the study
- Initiatives in many hospitals encouraging breastfeeding and disallowing the promotion of breast milk substitutes
- Reluctance of parents to bring their healthy babies to hospitals or doctor’s offices for the site visits generally required in infant formula study protocols
In our experience, decentralization addresses these challenges, enabling sponsors and CROs to cast a wide geographic net for eligible participants and reducing study burden on parents to encourage enrollment and enhance retention.
In Decentralized Trials, You Can Never Be Over-Prepared
Since 2016, Premier has supported 5 infant formula studies, including one successfully completed hybrid decentralized clinical trial (DCT), and more than 800 babies enrolled. Based on lessons learned from our experience with decentralization approaches, we have developed tools and best practices to overcome enrollment challenges and accelerate study start-up in decentralized infant formula trials.
1. Keep the smallest of details in mind, starting with strict inclusion and exclusion criteria.
Careful consideration of inclusion and exclusion criteria helps limit screen failures. For example, for studies of cow’s milk-based infant formulas, it is useful to include prior consumption of—and ability to tolerate—such formulas in the inclusion criteria and any personal or immediate family history of cow’s milk protein allergy or intolerance in the exclusion criteria. It is also important to adhere to enrolling babies who measure within the 5th and 95th percentile for length and weight according to the World Health Organization (WHO) growth chart.
2. Get social. Leverage the influence of social media advertising to build a pipeline of potential subjects.
For infant formula trials, it takes a lot of leads to get the number of randomized babies necessary to complete enrollment. Traditional patient recruitment firms are not effective for these studies as pregnant women do not identify as “sick.” Instead, early digital marketing efforts, consisting of targeted ads with a focus on metropolitan areas with high birth rates, are required to fill the lead funnel.
At Premier, we have tested all the major social platforms and found those that are most effective for reaching pregnant women who know they will not breastfeed. Ads on these platforms take interested individuals to a landing page with study-specific screening questions that offers them the opportunity to self-select for participation. In addition to having expertise in developing marketing playbooks for institutional review board (IRB) approval and use internally as well as by external agencies, our in-house marketing staff is adept at content development and ad placement for sponsors who choose to allow Premier to manage marketing efforts to reduce spend and increase flexibility.
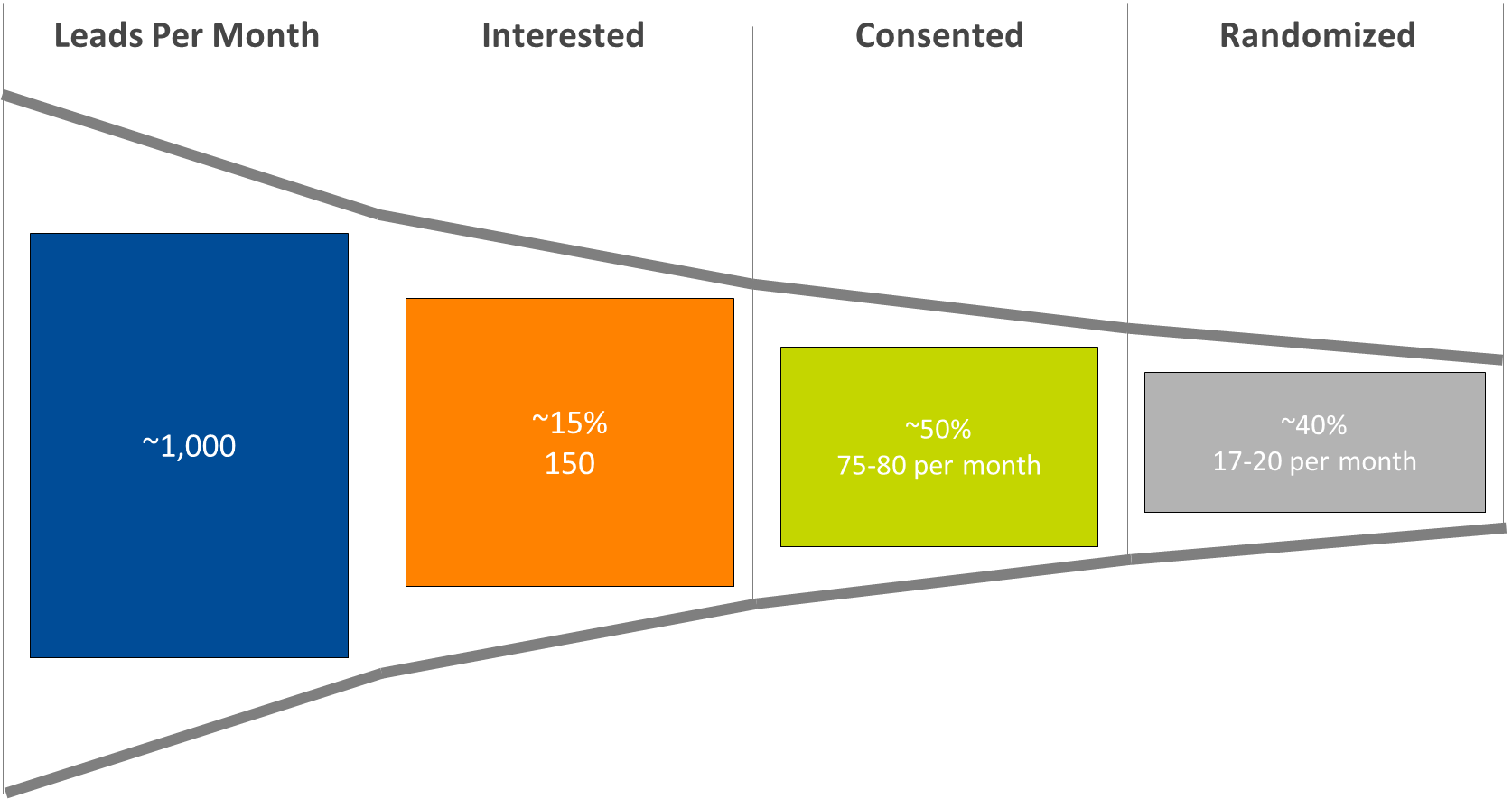
Based on our previous experience in recruiting for a decentralized infant formula trial in 10 metropolitan areas, enrollment rate is directly related to marketing spend. About 1,000 leads need to be generated each month to randomize 17-20 subjects, with a resulting spend of approximately $1,000 per randomized subject and an estimated 80% of randomized subjects completing the study.
Figure 1. Subject identification funnel: from lead generation to randomization
3. Keep clarity at the forefront of study training, and reinforce through telehealth.
Anthropometrics—including measurements of weight, length, and head circumference—are integral to outcomes assessments in infant formula studies. A potential concern with decentralization is increased variability in anthropometry based on the individual performing the measurements. In our experience, the use of home healthcare nurses to perform anthropometrics did not necessarily reduce variability. Instead, training parents or caregivers to perform anthropometrics while on a telehealth visit with virtual site staff monitoring measurement performance not only increased parent/caregiver confidence but also increased consistency. Developing training videos—and making viewing of those videos part of consent—helps parents understand exactly what they are signing up for and provides them with reassurance that they can manage the responsibilities of study participation.
4. DCTs will fail without the right technologies. Deploy them, simply.
Finding the right vendors for electronic patient reported outcomes (ePROs), eDiaries, interactive response technology (IRT), electronic data capture (EDC), remote monitoring, and data management is critical to success in DCT studies. Direct data entry capability is necessary, and ePRO must be easy for parents to use. At Premier, our IRT system is pre-programmed to include the following parameters, and we recommend this approach for any infant formula study to ensure accurate randomization.
- WHO growth parameters and charts by gender and age in days to ensure accuracy at the time of enrollmentExpiration dates for supply of both the investigational and comparator formulas.
Remarque, our in-house study management technology platform can be used to integrate all study-related data—including administrative and financial, study management, patient, and third-party data—in one place to facilitate in-process analytics, reports, and metrics. This is particularly key for enabling the timely review of growth charts, ensuring patient safety throughout the life of the study.
5. For participants, it’s the little things that make a big difference. Continual engagement is key.
Navigating the journey from expressing interest to actually enrolling in a clinical trial takes time, so keeping in contact with parents and caregivers throughout the process is essential. At Premier, our parent support personnel routinely liaise with interested mothers via email, text, or phone calls to maintain their interest in the study and to troubleshoot any issues that arise during the consent process and after randomization. With this high level of support, study teams build strong relationships with parents that continue beyond the end of the study.
Implementation Made Easier with the Right Partner
Using these 5 strategies in tandem reduces enrollment timelines, increases subject retention, and accelerates study start-up, keeping trials moving forward to generate the data necessary for regulatory submissions. With an experienced CRO at the helm to oversee IRT development and maintenance, daily central monitoring, vendor management, data management, statistical analysis, and clinical study report creation, full decentralization is not only possible, but preferable for all infant formula trials.
To learn how Premier’s innovative decentralized clinical trial model can help propel your infant formula study, contact us.
[1] Helfer B, et al. Conduct and reporting of formula milk trials: a systematic review. BMJ. 2021;375:n2202.
[2] U.S. Food and Drug Administration. FDA Developing New Framework for Continued, Expanded Access to Infant Formula Options for U.S. Parents and Caregivers, July 6, 2022.