Molecular diagnostics can provide a more precise and scientifically sound basis for the practice of medicine. However, barriers to their adoption may increase the risk for developers and delay the practice of precision medicine. With the recent evolution of regulations governing investigational diagnostics, it has become vitally important for diagnostics developers to understand how to design and conduct trials that demonstrate the clinical utility of their tests to healthcare payers, regulators, and physicians.
This blog series covers ways to approach designing and conducting a randomized clinical trial (RCT) to demonstrate clinical utility.
For RCTs to demonstrate clinical utility, forethought is key
As a developer, you can increase the likelihood of successfully demonstrating clinical utility by weighing certain factors that impact the use of your molecular diagnostics device. For example, finding ways to reduce variability in physician decision-making or increase patient adherence with recommended treatments can help you save time and cost. Lay the foundation for demonstrating clinical utility by identifying:
- The diagnostic’s intended use, proposed indication, and target patient population
- The clinical decisions that are expected to result in improved health outcomes
- The physicians who make relevant treatment decisions and the context in which they make them
- Evidence for linkage between such treatment decisions and clinical outcomes
- Any accepted surrogate endpoints for the desired clinical outcome
To understand more about how these considerations can inform your RCT strategy, read Part I of this blog series.
1. Understand the requirements for an RCT to demonstrate clinical utility
The sequence of studies designed to demonstrate the precision, reproducibility, and analytic validity of a diagnostic differs from the Phase 1-2-3 model central to demonstrating the safety and efficacy of an investigational drug. However, the worlds of diagnostics and drugs converge in clinical utility studies. If unfamiliar with the intricacies of drug efficacy and safety studies, diagnostics developers may underestimate the challenges of demonstrating improved outcomes.
Factors that are key in demonstrating the safety and efficacy of therapeutics may or may not be relevant in RCTs of clinical utility. The requirements vary depending upon the indication. Important considerations include: protocol feasibility, site feasibility, investigator training, variability in physician decision-making, and participant adherence with the study regimen.
2. Perform rigorous protocol feasibility
For trial success, it is essential to solicit investigator feedback to ensure the feasibility of the study protocol and the operational plans to demonstrate your investigative molecular diagnostic’s clinical utility. To ensure that the protocol is practicable in the field, you must consider conditions that affect the ability of sites to execute the study and their interest in participating.
The protocol feasibility process should account for:
- Patient demography, disease epidemiology, standards of care, and treatment pathways
- Investigator interest and design acceptance based on the protocol outline
- Site enrollment capabilities
- Investigator-reported enrollment data in light of previous experience and published data
- The competitive landscape
- Enrollment scenario modeling in accordance with feasibility findings and sponsor specifications
- The ideal site profile for successful trial conduct
3. Preform Rigorous Site Feasibility
Investigative site selection is critical in any study of health outcomes. Sponsors often select sites based on obvious practical criteria such as availability of patients in the target population, adequate procedure volume to provide samples for analysis, and support for the required sample flow (for both the investigative diagnostic and the comparator). While these are all important, additional site selection factors apply in a clinical utility study.
One is investigator experience in studies of safety and efficacy. An investigator learning curve in multiple aspects of clinical trials is well documented. For example, less experienced investigators perform less well in securing informed consent, time to first-patient-in, enrollment rate, pharmacokinetic/pharmacodynamic sampling, and protocol adherence.
In addition to developing an ideal site and investigator profile, a rigorous site feasibility process will hinge on strict criteria for candidate selection.
Multi-step process for optimal site selection
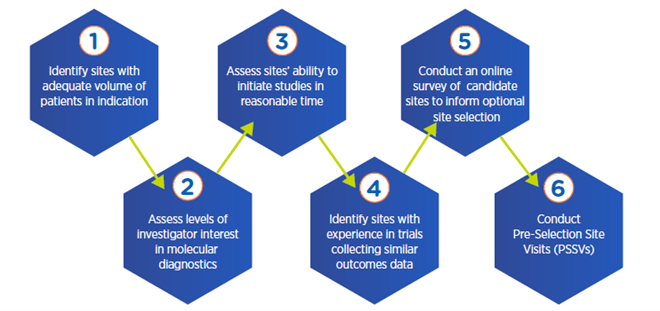
4. Analyze potential variability in physician decision-making
Diagnostics exist to enable decision-making. However, few diagnostics developers realize the potential ramifications of variability in physician decision-making, whether in investigator interpretation of diagnostics results or in physicians’ therapeutic choices. Both can complicate determination of improved outcomes linked to a new diagnostic.
What happens when demonstrating an improved clinical outcome depends on a physician’s choice from an array of treatment regimens with different safety, efficacy, and tolerability profiles? For example, in oncology, when there is considerable flexibility within the standard of care, physicians may base treatment preferences on previous outcomes they’ve observed or on a perceived risk-benefit balance for a particular patient.
Many medical centers are incorporating Clinical Decision Support Systems (CDSS), which can assist with clinical guidelines, automated alerts and reminders, recommended indication-specific orders, and other decision-support modalities. By providing investigator training and CDSS to investigative sites, you may be able to reduce variability in physician decision-making, preventing inconsistencies from masking improvements associated with use of the new diagnostic being evaluated.
5. Proactively address participant adherence during the RCT
Patient adherence is a significant consideration in clinical utility studies for precision medicine and can make it difficult to demonstrate a benefit of one diagnostic over another or to demonstrate treatment benefit. Patients may be non-adherent with follow-up, treatment, and/or the screening process itself.
Data suggests approximately 50 percent of patients fail to continue medication as prescribed. In oncology, with treatments that carry significant quality-of-life ramifications, such as undesirable hormonal effects of breast and prostate cancer treatments, patients can be strikingly non-adherent.
In planning a clinical utility study, review the literature on non-adherence in the relevant disease state and seek out instruments and technologies that sites can use to track and encourage adherence. For example, simple methods such as checking prescription bottle dates or use of a validated adherence questionnaire might be combined with advanced technologies that generate automated reminders via text messaging, or “smart” pills that transmit a signal confirming ingestion. These strategies can help safeguard your ability to demonstrate treatment benefit in connection with your diagnostic device.
Get the help you need to demonstrate the clinical utility of your novel molecular diagnostic
Demonstrating the clinical utility of a new molecular diagnostic for healthcare payers, regulators, and physicians is a challenge that requires expertise in designing and conducting studies of both diagnostics and therapeutics. Diagnostics developers need specific knowledge and skills to plan and execute successful strategies, including RCTs, that demonstrate improved outcomes over a comparator.
With extensive experience and expertise in diagnostics studies and drug studies, Premier Research can help. We have conducted many molecular diagnostics clinical utility studies demonstrating improved outcomes over a comparator and have supported successful 510(k) and PMA submissions. Consult our in-house clinical and biostatistical experts for efficient planning, design, and conduct of cost-effective programs to demonstrate clinical utility of your new molecular diagnostic.
Contact Premier Research for efficient strategies and assistance to demonstrate the clinical utility of your molecular diagnostic, decrease risk, and advance precision medicine.




