Real-world data (RWD) and real-world evidence (RWE) have become an increasingly important part of the medical product development landscape. These tools can enable sponsors to optimize clinical trial designs and demonstrate the real-world effectiveness of new medical products. Part 2 of this three-part blog series outlines a fit-for-purpose approach to RWE.
(See Part 1: What is Real-World Data and Evidence and How It Can Facilitate Clinical Development.)
Expert consultation helps clarify whether a program lends itself to RWD/RWE
With RWE, every situation demands its own strategy. Expert guidance can help sponsors make critical decisions about using RWD and RWE.
Sponsors need to evaluate if their RWE strategies reduce time and costs while generating meaningful outcomes without incurring undue risk. Premier experts help sponsors determine whether an RWE approach will favor their overall development plans through gap analyses and other assessments within our fit-for-purpose model. If so, they will help them implement it and analyze the results.
Criteria to evaluate an RWE strategy include:
- The robustness of existing data
To address the key questions a sponsor is investigating — often related to healthcare outcomes or costs —determine how much data has already been generated. To make up for a lack of relevant data; leveraging RWE in support may make sense if high-quality data sources can supplement that gap. - The end goal
Understanding the sponsor’s reasons for considering an RWE strategy is vital in deciding whether it is advisable. Is the goal to gain insights for a better protocol? Is it to develop a regulatory strategy based on RWE? - The target population’s accessibility
If the target population isn’t associated with a large trove of accessible data, then RWD may not be a viable pathway. However, if the population is diabetic, for example, or has high blood pressure, there’s a wealth of existing data that can be mined. - How regulators have responded to similar applications
For due diligence, the FDA CDRH document, Examples of Real-World Evidence (RWE) Used in Medical Device Regulatory Decisions1, presents a broad selection of applications RWE has supported.
Fitting RWD into your data strategy
Below are three ways RWD might contribute to an overall data strategy to demonstrate that a treatment is safe and effective for public use.
- RWE can supplement existing data
Supplementing clinical study results with real-world evidence can help when experimental data could be stronger. For example, a company with 100,000 patients’ worth of real-world data collected outside the U.S. is submitting a package to the FDA. They include their real-world evidence with the U.S. clinical trial evidence to enhance the overall package. - RWE can eliminate the need to repeat existing studies
Currently, the 505(b)(2) is the pathway most suited to the integration of real-world evidence. For drugs that have already been approved, sponsors can acquire FDA approval for a different indication or patient population without repeating all the work, such as safety studies, required for an NDA. - RWE can improve clinical trial design
A third way real-world evidence can enhance a data strategy is by enabling better-structured data design — better clinical trials design. By identifying the right population, sponsors will be able to locate them more efficiently, hastening recruitment.
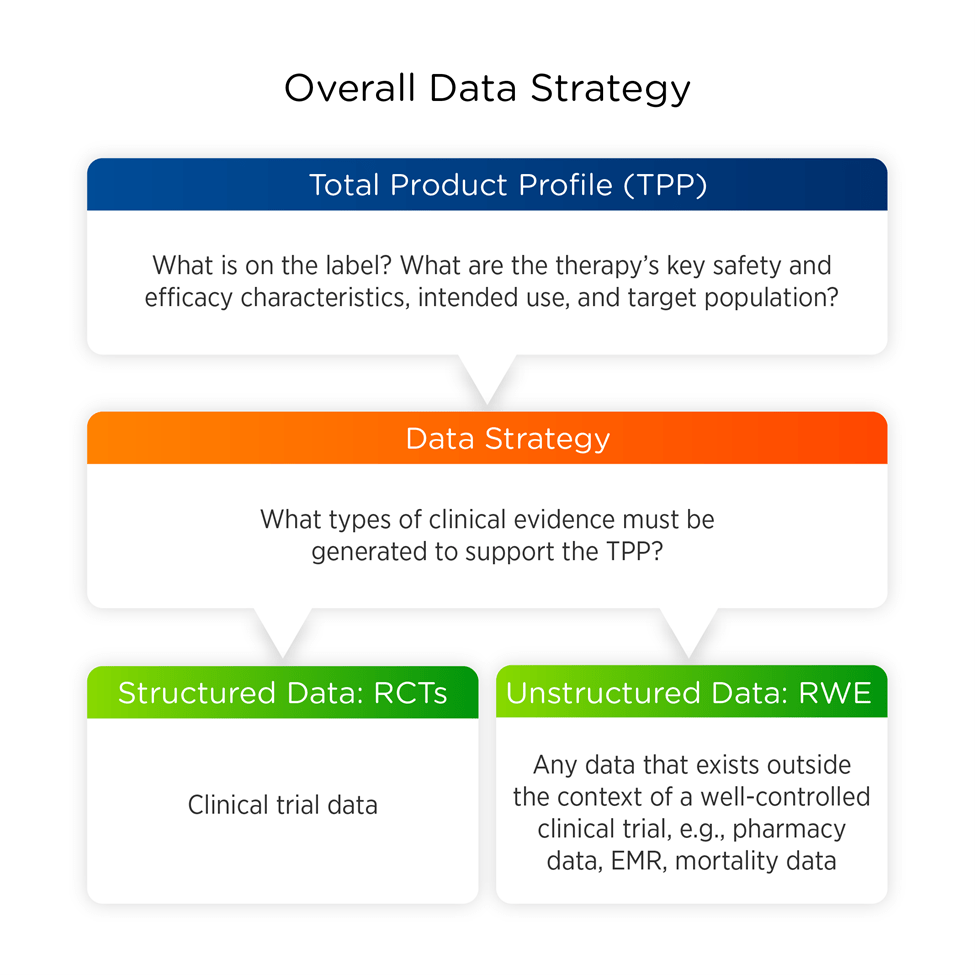
Figure 1. How real-world evidence (RWE) supports an overall data strategy.
Once the target product profile (TPP) is identified, we can determine the data strategy required to support it (Figure 3). This strategy may include structured data (clinical trial data) and unstructured data (RWD). Utilizing a tokenized platform such as DataVant helps maintain data quality, completeness, and privacy by linking all of each patient’s assorted, unstructured data elements under one unique, anonymous identifier.
Key Takeaways
Expert guidance helps sponsors make critical planning decisions around the use of RWD and RWE to ensure that RWE strategies reduce time and costs without incurring undue risk. RWE contributes by:
- Supporting existing data
- Eliminating the need to repeat existing studies
- Improving clinical trial design
Premier’s fit-for-purpose model entails gap analyses and other assessments to determine whether and if so, how, RWD and RWE can support customer data strategies. Click here to explore our expertise and how we can help sponsors make critical decisions about incorporating RWE into their data strategy.
[1] Examples of Real-World Evidence (RWE) Used in Medical Device. U.S. Food & Drug Administration. www.fda.gov/media/146258/download. Published February 26, 2021. Accessed May 18, 2022.




