Last Updated: May 28, 2025, 10 am UTC
For researchers who are developing or working with analytical procedures, the new ICH Q14 Analytical Procedure Development and revised ICH Q2(R2) Validation of Analytical Procedures drafts represent foundational guidance documents. Together, they describe suggested development and validation activities for the lifecycle of an analytical procedure used to assess the quality of drug substances and drug products. ICH Q14 describes scientific principles and risk-based approaches for developing and maintaining suitable analytical procedures, while ICH Q2(R2) provides guidelines for establishing, submitting, and maintaining evidence that an analytical procedure is fit for purpose (assuring drug quality).
The target date for finalization and implementation of these documents in the local regional regulatory system is May 2023; this two-part blog series explains everything you need to know to prepare. (Read Part 1 here.)
Overview of ICH Q2(R2)
ICH Q2(R2) discusses elements to consider while validating analytical procedures that will be part of registration applications, including what data should be presented. More specifically, ICH Q2(R2) provides recommendations on how to derive and evaluate the various validation tests required for each type of analytical procedure, operationalizing the development plan outlined by ICH Q14.
Table 1. Descriptions of Key Sections of ICH Q2(R2)
| Section | Updated or New | Description |
| Introduction | Updated | Describes the objective of the guideline |
| Scope | Updated | Outlines how the guideline should be applied and aligns with ICH Q14 |
| Analytical Procedure Validation Study | Updated | |
| Validation during the lifecycle of an analytical procedure | New | Provides validation approaches for different stages of the analytical procedure lifecycle (see Table 4) |
| Reportable Range | Updated | Offers expected reportable ranges for common uses of analytical procedures |
| Demonstration of stability indicating properties | New | Provides guidance on how to demonstrate the specificity or selectivity of stability-indicating tests |
| Considerations for multivariate analytical procedures | New | Describes factors to consider in calibrating and validating multivariate analytical procedures |
| Validation Tests, Methodology, and Evaluation | Updated | |
| Specificity / Selectivity | Updated | Describes requirements for demonstrating specificity or selectivity |
| Working Range | Updated | Provides approaches for establishing a specific working range |
| Accuracy and Precision | Updated | Describes how to evaluate and establish accuracy and precision |
| Robustness | Updated | Provides considerations for evaluating the suitability of the analytical procedure within the intended operating environment |
| Annex 1 | New | Offers guidance of how to select validation tests based on the objective or main performance characteristic of the analytical procedure |
| Annex 2 | New | Provides illustrative examples for common analytical techniques |
As part of the revision, ICH Q2 has been modernized to include newer technologies and serve as a collection of terms and their definitions. Designed to bridge the differences that exist among the various compendia and documents of ICH member regulatory authorities, the guideline also incorporates principles described in ICH Q8-Q10, which did not exist when Q2(R1) was drafted.
Table 2. Typical Performance Characteristics and Related Validation Tests
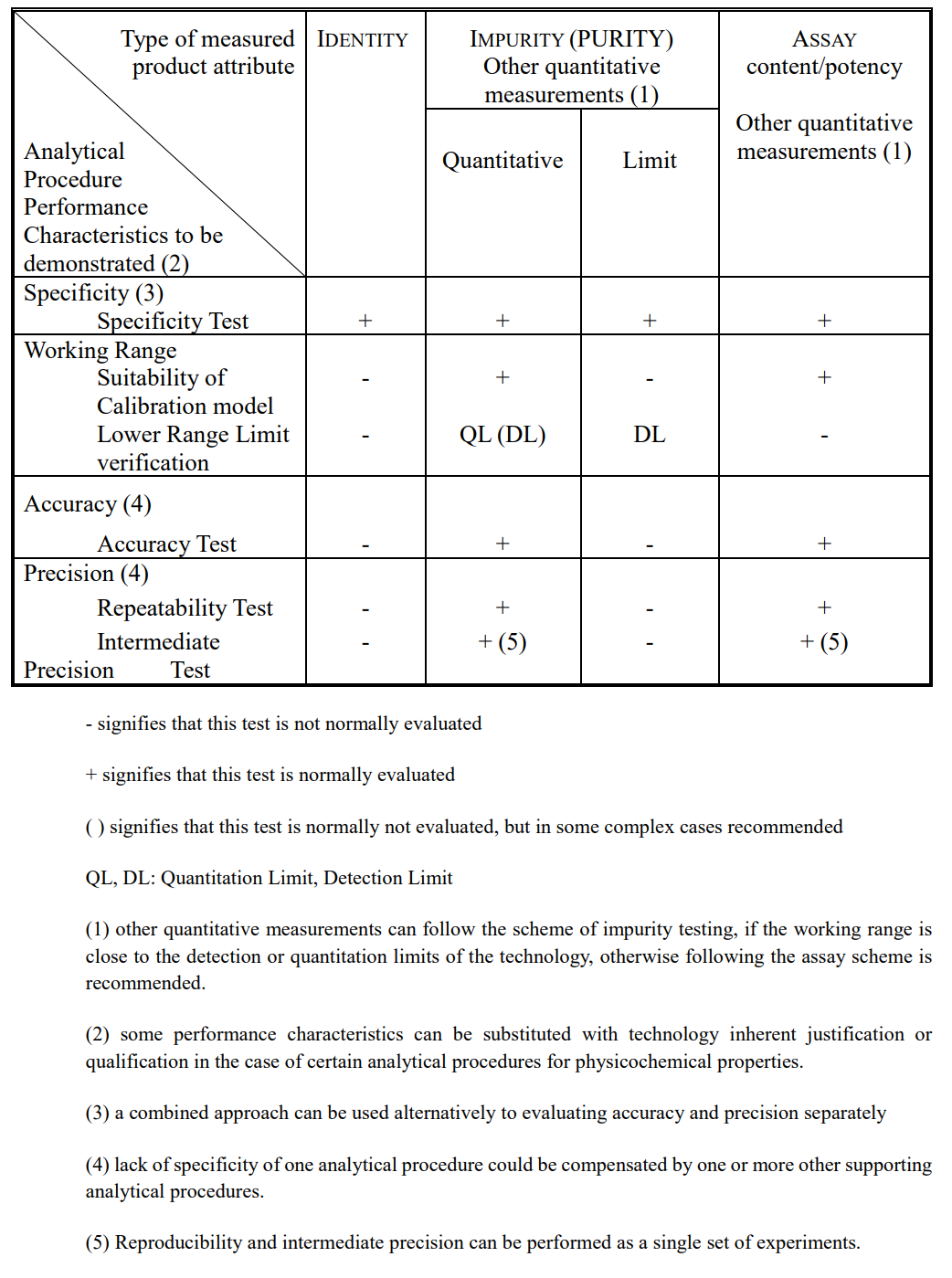
Source: ICH Q2(R2)
ICH Q2(R2) encourages the use of more advanced analytical procedures, with an expected downstream benefit of more robust quality oversight by drug manufacturers. Another expected benefit is an improvement in the adequacy of validation data submitted, resulting in fewer information requests and responses, which can delay application approval.
Parting Thoughts
Together, ICH Q14 and Q2(R2) establish harmonized scientific and technical principles for analytic procedure development over the entire procedure and product lifecycle. Importantly, the Q14 and Q2(R2) guidelines should be considered and applied in conjunction with other existing and prospective ICH “Q” guidelines. At Premier Research, we have followed the development and drafting of these two documents closely and have the expertise to help researchers put these guidelines into practice. To learn more about how we can help you increase the robustness of your analytical procedures, contact us.
Authors:
Constance Morris
Manager, Regulatory Affairs CMC
Ankita Rohishaliya
Manager, Regulatory Affairs CMC
References:
- International Council for Harmonisation. ICH Harmonised Guideline: Analytical Procedure Development Q14. Available at https://database.ich.org/sites/default/files/ICH_Q14_Document_Step2_Guideline_2022_0324.pdf.
- International Council for Harmonisation. ICH Harmonised Guideline: Validation of Analytical Procedures Q2(R2). Available at https://database.ich.org/sites/default/files/ICH_Q2-R2_Document_Step2_Guideline_2022_0324.pdf.

 Webinar
Webinar 


 Perspectives Blog
Perspectives Blog 