Clinical Research and Development
Helping biotech and specialty pharma companies navigate the complexities of clinical studies.
PREMIER PERSPECTIVE
Product Development Checklist: Considerations for Each Stage of the Drug Development Process
Developing a new biopharmaceutical product is a lengthy, high-stakes journey. A comprehensive plan and the right regulatory and therapeutic expertise can significantly accelerate the development timeline and increase the likelihood of marketing success.
PREMIER PERSPECTIVE
Advancing from Research to Development: What Can Go Wrong?
The drug development process is a long journey, beginning with drug discovery, moving through nonclinical and clinical studies, and ultimately culminating in regulatory approval.
A focus on end-to-end clinical research and development
Expert guidance, every step along the way
We’ve unified the disciplines that underpin successful clinical research and development, from dedicated rapid study start-up staff to Premier-trained project managers and fast-track application expertise. With so much at stake – livelihoods, expectant investors, and most important, the patients who entrust to us their health and safety – there is no room for shortcuts.
Our experienced research professionals are capable of managing even the most complex clinical research studies from Phase 1 to Phase 4. Our therapeutically focused teams have the knowledge and experience necessary to effectively manage situations as they arise, and our approach is firmly grounded in comprehensive project management principles and well-defined processes.
Service Areas:
Related Capabilities
Adding value at every step in the process, we provide expertise in strategic product development, global regulatory consulting, and technology applications to ensure data integrity, process efficiency, and timely analytics and reporting.
Strategic Product Development

Global Regulatory Consulting

Premier Ecosystem

Therapeutic Focus
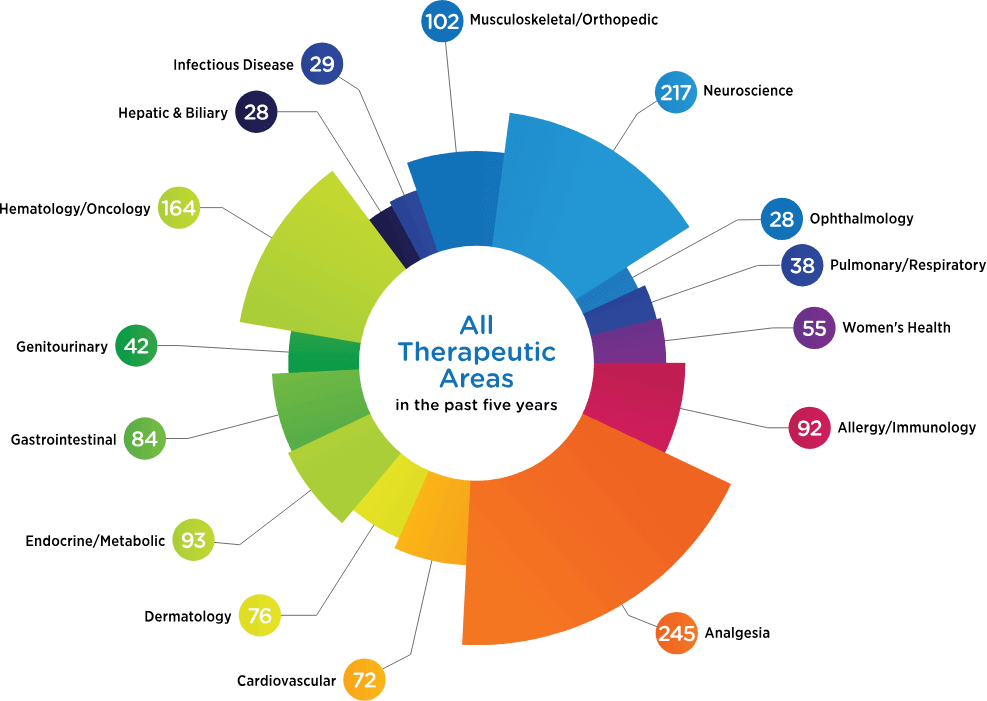
Therapeutic Focus
There’s no substitute for experience. And we have a lot of it. See what we’ve been busy doing for the past five years.
Check out our resource center
Our experts have developed an extensive library of white papers, case studies, blogposts, and other informative resources.
PREMIER INSIGHTS
WHITE PAPERS
WEBINARS
VIDEOS
PODCASTS
Connect with us
Ready to get started? So are we. Drop us a line to learn more about how we can help.