When selecting a CRO partner, sponsors usually focus on organizational experience – how long the company has been in business, its areas of therapeutic expertise and the breadth and depth of its service offerings. But, sponsors sometimes forget to ask about specific team experience. What do you know about the core study team members – the project manager, clinical manager, investigative site manager and clinical research associates (CRAs) – who might be working on your project?
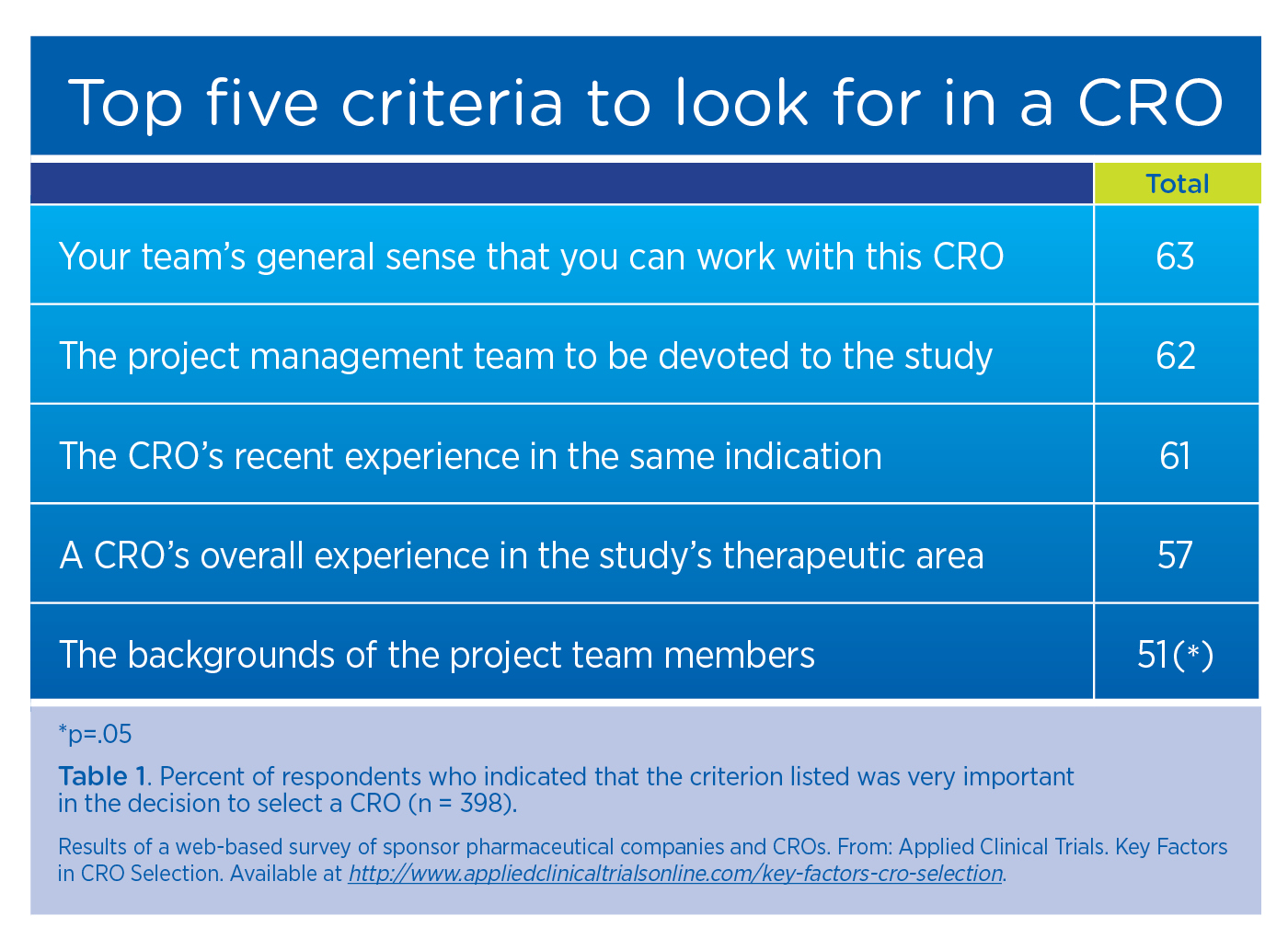
While it’s important to ask about organizational experience, team experience may be even more relevant to the success of a particular study. You want to work with a knowledgeable team that has a solid track record of providing services from protocol design to final study reporting. Unfortunately, the CRO industry is competitive and the turnover rate is high. When employees leave a CRO, they take their hands-on experience and intellectual capital with them. That’s why evaluating the team members assigned to your study is a critical part of the CRO selection process.
Here are some factors to consider when evaluating team experience:
- Expertise in the therapeutic area and disease indication
A strong background in the therapeutic area, disease indication, and target patient population will help study staff get up to speed more quickly on study design, recruitment strategies and the data collection and analysis tools to be used. Previous experience with the disease indication and its associated standard of care in the countries where the study may be conducted is also extremely beneficial. Existing treatment patterns and guidelines for the disease of interest may vary widely, and the specialist and site type may differ, as well. These factors can have a significant impact site and investigator selection, as well as the potential for patient recruitment. Working with a study team that is already familiar with these nuances can help to expedite the clinical trial development process. - Experience with studies in out-of-the-ordinary or complex indications
This is particularly important in rare or ultra-rare disease indications and pediatric studies, where experience in them is rare as well. When there is a very limited pool of eligible patients, you need to know the team can quickly learn the unique nuances of a patient population and their treatment which may often require specialized processes due to increased regulatory requirements, enhanced scrutiny and the challenges associated with recruitment and retention in these vulnerable populations. - Record of working with regulatory agencies worldwide
The regulatory and ethical framework for clinical trials may differ from country to country, or even among regions within a country. If the trial includes global sites, ask about the team’s experience in navigating the regulatory guidelines and ethical requirements in foreign countries. Other countries may also have complicated import/export requirements, especially with regard to the shipment of investigational products, so having a team that already knows these rules can give the study a leg up. - Critical thinking and problem-solving skills
Managing a clinical study is a complex process. Study team members, from project managers to CRAs, need to understand how to solve problems as they arise and when to involve the sponsor, all while protecting the regulatory stringency of the trial. - Experience dealing with sites that have never participated in industry-sponsored research
When sites or investigators do not have previous experience with industry-sponsored research, sponsors need to rely on the study team to deliver thorough and effective training. Hands-on experience in dealing with sites that are new to industry-sponsored research helps ensure that sites/investigators have a comprehensive understanding of the study requirements, as well as the processes need to maintain compliance and data quality. Having study team members who are practiced in the art of in-depth study training will make it easier to onboard and open effective sites. - Team chemistry
Collaboration among team members, from those in business development and clinical operations to those in medical, scientific and regulatory affairs, is an essential ingredient in clinical trial success. How well does the team communicate with each other? Have they worked together before?As a sponsor, don’t forget to assess your own team’s general sense that they will be able to work with the CRO team. These teams will need to work closely together for an extended period time, so a positive sponsor-CRO project team relationship is a must. - Confidence
In order to be effective, study team members need to be able to provide confident guidance to key opinion leaders (KOLs), investigators and site staff. They may also need to be able to negotiate the complexities of large academic institutions without feeling intimidated.
Having the right study team members can help to streamline a clinical trial and even improve its cost effectiveness. In addition, having a knowledgeable team of professionals with hands-on experience at the helm of your study is central to mitigating risk.
